Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study
- PMID: 21723781
- PMCID: PMC3148430
- DOI: 10.1016/S1470-2045(11)70149-8
Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study
Abstract
Background: The effects of extra-pleural pneumonectomy (EPP) on survival and quality of life in patients with malignant pleural mesothelioma have, to our knowledge, not been assessed in a randomised trial. We aimed to assess the clinical outcomes of patients who were randomly assigned to EPP or no EPP in the context of trimodal therapy in the Mesothelioma and Radical Surgery (MARS) feasibility study.
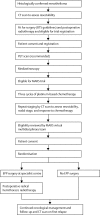
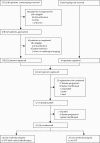
Methods: MARS was a multicentre randomised controlled trial in 12 UK hospitals. Patients aged 18 years or older who had pathologically confirmed mesothelioma and were deemed fit enough to undergo trimodal therapy were included. In a prerandomisation registration phase, all patients underwent induction platinum-based chemotherapy followed by clinical review. After further consent, patients were randomly assigned (1:1) to EPP followed by postoperative hemithorax irradiation or to no EPP. Randomisation was done centrally with computer-generated permuted blocks stratified by surgical centre. The main endpoints were feasibility of randomly assigning 50 patients in 1 year (results detailed in another report), proportion randomised who received treatment, proportion eligible (registered) who proceeded to randomisation, perioperative mortality, and quality of life. Patients and investigators were not masked to treatment allocation. This is the principal report of the MARS study; all patients have been recruited. Analyses were by intention to treat. This trial is registered, number ISRCTN95583524.
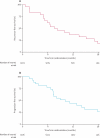
Findings: Between Oct 1, 2005, and Nov 3, 2008, 112 patients were registered and 50 were subsequently randomly assigned: 24 to EPP and 26 to no EPP. The main reasons for not proceeding to randomisation were disease progression (33 patients), inoperability (five patients), and patient choice (19 patients). EPP was completed satisfactorily in 16 of 24 patients assigned to EPP; in five patients EPP was not started and in three patients it was abandoned. Two patients in the EPP group died within 30 days and a further patient died without leaving hospital. One patient in the no EPP group died perioperatively after receiving EPP off trial in a non-MARS centre. The hazard ratio [HR] for overall survival between the EPP and no EPP groups was 1·90 (95% CI 0·92-3·93; exact p=0·082), and after adjustment for sex, histological subtype, stage, and age at randomisation the HR was 2·75 (1·21-6·26; p=0·016). Median survival was 14·4 months (5·3-18·7) for the EPP group and 19·5 months (13·4 to time not yet reached) for the no EPP group. Of the 49 randomly assigned patients who consented to quality of life assessment (EPP n=23; no EPP n=26), 12 patients in the EPP group and 19 in the no EPP group completed the quality of life questionnaires. Although median quality of life scores were lower in the EPP group than the no EPP group, no significant differences between groups were reported in the quality of life analyses. There were ten serious adverse events reported in the EPP group and two in the no EPP group.
Interpretation: In view of the high morbidity associated with EPP in this trial and in other non-randomised studies a larger study is not feasible. These data, although limited, suggest that radical surgery in the form of EPP within trimodal therapy offers no benefit and possibly harms patients.
Funding: Cancer Research UK (CRUK/04/003), the June Hancock Mesothelioma Research Fund, and Guy's and St Thomas' NHS Foundation Trust.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Surgery for mesothelioma? The debate continues.Lancet Oncol. 2011 Aug;12(8):713-4. doi: 10.1016/S1470-2045(11)70179-6. Epub 2011 Jun 30. Lancet Oncol. 2011. PMID: 21723780 No abstract available.
-
The MARS feasibility trial: conclusions not supported by data.Lancet Oncol. 2011 Nov;12(12):1093-4; author reply 1094-5. doi: 10.1016/S1470-2045(11)70307-2. Lancet Oncol. 2011. PMID: 22041539 No abstract available.
References
-
- Peto J, Hodgson JT, Matthews FE, Jones JR. Continuing increase in mesothelioma mortality in Britain. Lancet. 1995;345:535–539. - PubMed
-
- Sugarbaker DJ, Flores RM, Jaklitsch MT. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999;117:54–63. - PubMed
-
- Rusch VW, Rosenzweig K, Venkatraman E. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;22:788–795. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

