Transfer of arginine into lipid bilayers is nonadditive
- PMID: 21723820
- PMCID: PMC3127173
- DOI: 10.1016/j.bpj.2011.05.038
Transfer of arginine into lipid bilayers is nonadditive
Abstract
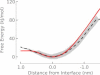
Computer simulations suggest that the translocation of arginine through the hydrocarbon core of a lipid membrane proceeds by the formation of a water-filled defect that keeps the arginine molecule hydrated even at the center of the bilayer. We show here that adding additional arginine molecules into one of these water defects causes only a small change in free energy. The barrier for transferring multiple arginines through the membrane is approximately the same as for a single arginine and may even be lower depending on the exact geometry of the system. We discuss these results in the context of arginine-rich peptides such as antimicrobial and cell-penetrating peptides.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Jiang Y., Lee A., MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. - PubMed
-
- Long S.B., Campbell E.B., Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. - PubMed
-
- Epand R.M., Vogel H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta. 1999;1462:11–28. - PubMed
-
- Richard J.P., Melikov K., Lebleu B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 2003;278:585–590. - PubMed
-
- Zorko M., Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv. Drug Deliv. Rev. 2005;57:529–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

