Methods in cardiomyocyte isolation, culture, and gene transfer
- PMID: 21723873
- PMCID: PMC3164875
- DOI: 10.1016/j.yjmcc.2011.06.012
Methods in cardiomyocyte isolation, culture, and gene transfer
Abstract
Since techniques for cardiomyocyte isolation were first developed 35 years ago, experiments on single myocytes have yielded great insight into their cellular and sub-cellular physiology. These studies have employed a broad range of techniques including electrophysiology, calcium imaging, cell mechanics, immunohistochemistry and protein biochemistry. More recently, techniques for cardiomyocyte culture have gained additional importance with the advent of gene transfer technology. While such studies require a high quality cardiomyocyte population, successful cell isolation and maintenance during culture remain challenging. In this review, we describe methods for the isolation of adult and neonatal ventricular myocytes from rat and mouse heart. This discussion outlines general principles for the beginner, but also provides detailed specific protocols and advice for common caveats. We additionally review methods for short-term myocyte culture, with particular attention given to the importance of substrate and media selection, and describe time-dependent alterations in myocyte physiology that should be anticipated. Gene transfer techniques for neonatal and adult cardiomyocytes are also reviewed, including methods for transfection (liposome, electroporation) and viral-based gene delivery.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
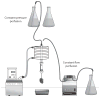

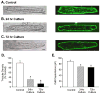
References
-
- Claycomb WC, Palazzo MC. Culture of the terminally differentiated adult cardiac muscle cell: a light and scanning electron microscope study. Dev Biol. 1980;80:466–82. - PubMed
-
- Powell T, Twist VW. A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun. 1976;72:327–33. - PubMed
-
- Wolska BM, Solaro RJ. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. Am J Physiol. 1996;271:H1250–H1255. - PubMed
-
- Wolska BM, Kitada Y, Palmiter KA, Westfall MV, Johnson MD, Solaro RJ. CGP-48506 increases contractility of ventricular myocytes and myofilaments by effects on actin-myosin reaction. Am J Physiol. 1996;270:H24–H32. - PubMed
-
- Dias FA, Walker LA, Arteaga GM, Walker JS, Vijayan K, Pena JR, et al. The effect of myosin regulatory light chain phosphorylation on the frequency-dependent regulation of cardiac function. J Mol Cell Cardiol. 2006;41:330–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

