Effect of tranexamic acid in traumatic brain injury: a nested randomised, placebo controlled trial (CRASH-2 Intracranial Bleeding Study)
- PMID: 21724564
- PMCID: PMC3128457
- DOI: 10.1136/bmj.d3795
Effect of tranexamic acid in traumatic brain injury: a nested randomised, placebo controlled trial (CRASH-2 Intracranial Bleeding Study)
Abstract
Objective: To assess the effect of tranexamic acid (which reduces bleeding in surgical patients and reduces mortality due to bleeding in trauma patients) on intracranial haemorrhage in patients with traumatic brain injury.
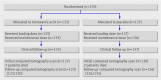
Methods: A nested, randomised, placebo controlled trial. All investigators were masked to treatment allocation. All analyses were by intention to treat. Patients 270 adult trauma patients with, or at risk of, significant extracranial bleeding within 8 hours of injury, who also had traumatic brain injury.
Interventions: Patients randomly allocated to tranexamic acid (loading dose 1 g over 10 minutes, then infusion of 1 g over 8 hours) or matching placebo.
Main outcome measures: Intracranial haemorrhage growth (measured by computed tomography) between hospital admission and then 24-48 hours later, with adjustment for Glasgow coma score, age, time from injury to the scans, and initial haemorrhage volume.
Results: Of the 133 patients allocated to tranexamic acid and 137 allocated to placebo, 123 (92%) and 126 (92%) respectively provided information on the primary outcome. All patients provided information on clinical outcomes. The mean total haemorrhage growth was 5.9 ml (SD 26.8) and 8.1 mL (SD 29.2) in the tranexamic acid and placebo groups respectively (adjusted difference -3.8 mL (95% confidence interval -11.5 to 3.9)). New focal cerebral ischaemic lesions occurred in 6 (5%) patients in the tranexamic acid group versus 12 (9%) in the placebo group (adjusted odds ratio 0.51 (95% confidence interval 0.18 to 1.44)). There were 14 (11%) deaths in the tranexamic acid group and 24 (18%) in the placebo group (adjusted odds ratio 0.47 (0.21 to 1.04)).
Conclusions: This trial shows that neither moderate benefits nor moderate harmful effects of tranexamic acid in patients with traumatic brain injury can be excluded. However, the analysis provides grounds for further clinical trials evaluating the effect of tranexamic acid in this population. Trial registration ISRCTN86750102.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Comment in
-
Tranexamic acid for traumatic brain injury.BMJ. 2011 Jul 1;343:d3958. doi: 10.1136/bmj.d3958. BMJ. 2011. PMID: 21724565 No abstract available.
References
-
- Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, McClelland B, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2007;17:CD001886. - PubMed
-
- CRASH-2 Trial Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23-32. - PubMed
-
- Bruns J Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia 2003;44:2-10. - PubMed
-
- CRASH Trial Collaborators. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet 2005;365:1957-9. - PubMed
-
- Oertel M, Kelly DF, McArthur D, Boscardin WJ, Glenn TC, Lee JH, et al. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg 2002;96:109-16. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical