Fine-tuning p53 activity through C-terminal modification significantly contributes to HSC homeostasis and mouse radiosensitivity
- PMID: 21724834
- PMCID: PMC3134085
- DOI: 10.1101/gad.2024411
Fine-tuning p53 activity through C-terminal modification significantly contributes to HSC homeostasis and mouse radiosensitivity
Abstract
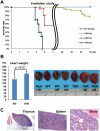
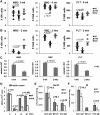
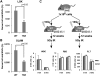
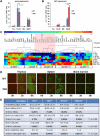
Cell cycle regulation in hematopoietic stem cells (HSCs) is tightly controlled during homeostasis and in response to extrinsic stress. p53, a well-known tumor suppressor and transducer of diverse stress signals, has been implicated in maintaining HSC quiescence and self-renewal. However, the mechanisms that control its activity in HSCs, and how p53 activity contributes to HSC cell cycle control, are poorly understood. Here, we use a genetically engineered mouse to show that p53 C-terminal modification is critical for controlling HSC abundance during homeostasis and HSC and progenitor proliferation after irradiation. Preventing p53 C-terminal modification renders mice exquisitely radiosensitive due to defects in HSC/progenitor proliferation, a critical determinant for restoring hematopoiesis after irradiation. We show that fine-tuning the expression levels of the cyclin-dependent kinase inhibitor p21, a p53 target gene, contributes significantly to p53-mediated effects on the hematopoietic system. These results have implications for understanding cell competition in response to stresses involved in stem cell transplantation, recovery from adverse hematologic effects of DNA-damaging cancer therapies, and development of radioprotection strategies.
Figures



References
-
- Appella E, Anderson CW 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem 268: 2764–2772 - PubMed
-
- Aranda-Anzaldo A, Dent MAR 2007. Reassessing the role of p53 in cancer and ageing from an evolutionary perspective. Mech Ageing Dev 128: 293–302 - PubMed
-
- Cheng T, Rodrigues N, Shen H, Yang Y-g, Dombkowski D, Sykes M, Scadden DT 2000. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287: 1804–1808 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
