Gamma-aminobutyric acid type B (GABA(B)) receptor internalization is regulated by the R2 subunit
- PMID: 21724853
- PMCID: PMC3129212
- DOI: 10.1074/jbc.M110.220814
Gamma-aminobutyric acid type B (GABA(B)) receptor internalization is regulated by the R2 subunit
Abstract
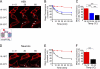
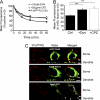
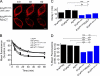
γ-Aminobutyric acid type B (GABA(B)) receptors are important for slow synaptic inhibition in the CNS. The efficacy of inhibition is directly related to the stability of cell surface receptors. For GABA(B) receptors, heterodimerization between R1 and R2 subunits is critical for cell surface expression and signaling, but how this determines the rate and extent of receptor internalization is unknown. Here, we insert a high affinity α-bungarotoxin binding site into the N terminus of the R2 subunit and reveal its dominant role in regulating the internalization of GABA(B) receptors in live cells. To simultaneously study R1a and R2 trafficking, a new α-bungarotoxin binding site-labeling technique was used, allowing α-bungarotoxin conjugated to different fluorophores to selectively label R1a and R2 subunits. This approach demonstrated that R1a and R2 are internalized as dimers. In heterologous expression systems and neurons, the rates and extents of internalization for R1aR2 heteromers and R2 homomers are similar, suggesting a regulatory role for R2 in determining cell surface receptor stability. The fast internalization rate of R1a, which has been engineered to exit the endoplasmic reticulum, was slowed to that of R2 by truncating the R1a C-terminal tail or by removing a dileucine motif in its coiled-coil domain. Slowing the rate of internalization by co-assembly with R2 represents a novel role for GPCR heterodimerization whereby R2 subunits, via their C terminus coiled-coil domain, mask a dileucine motif on R1a subunits to determine the surface stability of the GABA(B) receptor.
Figures


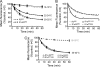


Similar articles
-
Sushi domains confer distinct trafficking profiles on GABAB receptors.Proc Natl Acad Sci U S A. 2012 Jul 24;109(30):12171-6. doi: 10.1073/pnas.1201660109. Epub 2012 Jul 9. Proc Natl Acad Sci U S A. 2012. PMID: 22778417 Free PMC article.
-
Tracking cell surface GABAB receptors using an alpha-bungarotoxin tag.J Biol Chem. 2008 Dec 12;283(50):34745-52. doi: 10.1074/jbc.M803197200. Epub 2008 Sep 23. J Biol Chem. 2008. PMID: 18812318 Free PMC article.
-
C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors.J Neurosci. 2001 Feb 15;21(4):1189-202. doi: 10.1523/JNEUROSCI.21-04-01189.2001. J Neurosci. 2001. PMID: 11160389 Free PMC article.
-
Signal transduction by GABA(B) receptor heterodimers.Neuropsychopharmacology. 2000 Oct;23(4 Suppl):S41-9. doi: 10.1016/S0893-133X(00)00145-7. Neuropsychopharmacology. 2000. PMID: 11008066 Review.
-
The metabotropic GABA receptor: molecular insights and their functional consequences.Cell Mol Life Sci. 2000 Apr;57(4):635-50. doi: 10.1007/PL00000725. Cell Mol Life Sci. 2000. PMID: 11130463 Free PMC article. Review.
Cited by
-
Modulation of cell surface GABA(B) receptors by desensitization, trafficking and regulated degradation.World J Biol Chem. 2012 Apr 26;3(4):61-72. doi: 10.4331/wjbc.v3.i4.61. World J Biol Chem. 2012. PMID: 22558486 Free PMC article.
-
Using an α-bungarotoxin binding site tag to study GABA A receptor membrane localization and trafficking.J Vis Exp. 2014 Mar 28;(85):51365. doi: 10.3791/51365. J Vis Exp. 2014. PMID: 24747556 Free PMC article.
-
Comparative expression study of the endo-G protein coupled receptor (GPCR) repertoire in human glioblastoma cancer stem-like cells, U87-MG cells and non malignant cells of neural origin unveils new potential therapeutic targets.PLoS One. 2014 Mar 24;9(3):e91519. doi: 10.1371/journal.pone.0091519. eCollection 2014. PLoS One. 2014. PMID: 24662753 Free PMC article.
-
A naturally occurring GIP receptor variant undergoes enhanced agonist-induced desensitization, which impairs GIP control of adipose insulin sensitivity.Mol Cell Biol. 2014 Oct 1;34(19):3618-29. doi: 10.1128/MCB.00256-14. Epub 2014 Jul 21. Mol Cell Biol. 2014. PMID: 25047836 Free PMC article.
-
Genome-scale identification of cellular pathways required for cell surface recognition.Genome Res. 2018 Sep;28(9):1372-1382. doi: 10.1101/gr.231183.117. Epub 2018 Jun 18. Genome Res. 2018. PMID: 29914970 Free PMC article.
References
-
- Enna S. J., Bowery N. G. (2004) Biochem. Pharmacol 68, 1541–1548 - PubMed
-
- Bowery N. G. (1989) Trends Pharmacol. Sci. 10, 401–407 - PubMed
-
- Bettler B., Kaupmann K., Mosbacher J., Gassmann M. (2004) Physiol. Rev. 84, 835–867 - PubMed
-
- Bowery N. G., Enna S. J. (2000) J. Pharmacol. Exp. Ther. 292, 2–7 - PubMed
-
- Marshall F. H., Jones K. A., Kaupmann K., Bettler B. (1999) Trends Pharmacol. Sci. 20, 396–399 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

