Mapping the substrate binding site of phenylacetone monooxygenase from Thermobifida fusca by mutational analysis
- PMID: 21724896
- PMCID: PMC3165276
- DOI: 10.1128/AEM.00687-11
Mapping the substrate binding site of phenylacetone monooxygenase from Thermobifida fusca by mutational analysis
Abstract
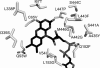
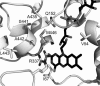
Baeyer-Villiger monooxygenases catalyze oxidations that are of interest for biocatalytic applications. Among these enzymes, phenylacetone monooxygenase (PAMO) from Thermobifida fusca is the only protein showing remarkable stability. While related enzymes often present a broad substrate scope, PAMO accepts only a limited number of substrates. Due to the absence of a substrate in the elucidated crystal structure of PAMO, the substrate binding site of this protein has not yet been defined. In this study, a structural model of cyclopentanone monooxygenase, which acts on a broad range of compounds, has been prepared and compared with the structure of PAMO. This revealed 15 amino acid positions in the active site of PAMO that may account for its relatively narrow substrate specificity. We designed and analyzed 30 single and multiple mutants in order to verify the role of these positions. Extensive substrate screening revealed several mutants that displayed increased activity and altered regio- or enantioselectivity in Baeyer-Villiger reactions and sulfoxidations. Further substrate profiling resulted in the identification of mutants with improved catalytic properties toward synthetically attractive compounds. Moreover, the thermostability of the mutants was not compromised in comparison to that of the wild-type enzyme. Our data demonstrate that the positions identified within the active site of PAMO, namely, V54, I67, Q152, and A435, contribute to the substrate specificity of this enzyme. These findings will aid in more dedicated and effective redesign of PAMO and related monooxygenases toward an expanded substrate scope.
Figures



References
-
- Alphand V., Furstoss R. 1992. Microbiological transformations. 22. Microbiologically mediated Baeyer-Villiger reactions—a unique route to several bicyclic gamma-lactones in high enantiomeric purity. J. Org. Chem. 57:1306–1309
-
- Bocola M., et al. 2005. Converting phenylacetone monooxygenase into phenylcyclohexanone monooxygenase by rational design: towards practical Baeyer-Villiger monooxygenases. Adv. Synth. Catal. 347:979–986
-
- Clouthier C. M., Kayser M. M., Reetz M. T. 2006. Designing new Baeyer-Villiger monooxygenases using restricted CASTing. J. Org. Chem. 71:8431–8437 - PubMed
-
- de Gonzalo G., Mihovilovic M. D., Fraaije M. W. 2010. Recent developments in the application of Baeyer-Villiger monooxygenases as biocatalysts. Chembiochem 11:2208–2231 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

