Glycomic analysis of human mast cells, eosinophils and basophils
- PMID: 21725073
- PMCID: PMC3230278
- DOI: 10.1093/glycob/cwr089
Glycomic analysis of human mast cells, eosinophils and basophils
Abstract
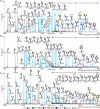
In allergic diseases such as asthma, eosinophils, basophils and mast cells, through release of preformed and newly generated mediators, granule proteins and cytokines, are recognized as key effector cells. While their surface protein phenotypes, mediator release profiles, ontogeny, cell trafficking and genomes have been generally explored and compared, there has yet to be any thorough analysis and comparison of their glycomes. Such studies are critical to understand the contribution of carbohydrates to the induction and regulation of allergic inflammatory responses and are now possible using improved technologies for detecting and characterizing cell-derived glycans. We thus report here the application of high-sensitivity mass spectrometric-based glycomics methodologies to the analysis of N-linked glycans derived from isolated populations of human mast cells, eosinophils and basophils. The samples were subjected to matrix-assisted laser desorption ionization (MALDI) time-of-flight (TOF) screening analyses and MALDI-TOF/TOF sequencing studies. Results reveal substantive quantities of terminal N-acetylglucosamine containing structures in both the eosinophil and the basophil samples, whereas mast cells display greater relative quantities of sialylated terminal epitopes. For the first time, we characterize the cell surface glycan structures of principal allergic effector cells, which by interaction with glycan-binding proteins (e.g. lectins) have the possibility to dictate cellular functions, and might thus have important implications for the pathogenesis of inflammatory and allergic diseases.
Figures


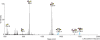

Similar articles
-
Lectins interact differentially with purified human eosinophils, cultured cord blood-derived mast cells and the myeloid leukaemic cell line AML14.3D10: induction of interleukin-4 secretion is conserved among granulocytes, but is not proportional to agglutination or lectin-glycoprotein interaction.Clin Exp Allergy. 2003 Jul;33(7):930-5. doi: 10.1046/j.1365-2222.2003.01625.x. Clin Exp Allergy. 2003. PMID: 12859449
-
Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils.J Allergy Clin Immunol. 2000 Jun;105(6 Pt 1):1093-100. doi: 10.1067/mai.2000.107127. J Allergy Clin Immunol. 2000. PMID: 10856141
-
5. IgE, mast cells, basophils, and eosinophils.J Allergy Clin Immunol. 2006 Feb;117(2 Suppl Mini-Primer):S450-6. doi: 10.1016/j.jaci.2005.11.016. J Allergy Clin Immunol. 2006. PMID: 16455345 Review.
-
Insights into the hyperglycosylation of human chorionic gonadotropin revealed by glycomics analysis.PLoS One. 2020 Feb 11;15(2):e0228507. doi: 10.1371/journal.pone.0228507. eCollection 2020. PLoS One. 2020. PMID: 32045434 Free PMC article.
-
The Expression and Function of CD300 Molecules in the Main Players of Allergic Responses: Mast Cells, Basophils and Eosinophils.Int J Mol Sci. 2020 Apr 30;21(9):3173. doi: 10.3390/ijms21093173. Int J Mol Sci. 2020. PMID: 32365988 Free PMC article. Review.
Cited by
-
N-linked glycan profiling of GGTA1/CMAH knockout pigs identifies new potential carbohydrate xenoantigens.Xenotransplantation. 2013 Sep-Oct;20(5):277-91. doi: 10.1111/xen.12047. Epub 2013 Sep 5. Xenotransplantation. 2013. PMID: 24033743 Free PMC article.
-
Glycobiology of Eosinophilic Inflammation: Contributions of Siglecs, Glycans, and Other Glycan-Binding Proteins.Front Med (Lausanne). 2017 Aug 2;4:116. doi: 10.3389/fmed.2017.00116. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28824909 Free PMC article. Review.
-
The architecture of the IgG anti-carbohydrate repertoire in primary antibody deficiencies.Blood. 2019 Nov 28;134(22):1941-1950. doi: 10.1182/blood.2019001705. Blood. 2019. PMID: 31537530 Free PMC article.
-
Analytical tools for the study of cellular glycosylation in the immune system.Front Immunol. 2013 Dec 11;4:451. doi: 10.3389/fimmu.2013.00451. Front Immunol. 2013. PMID: 24376449 Free PMC article. Review.
-
N-Glycomics of Human Erythrocytes.Int J Mol Sci. 2021 Jul 28;22(15):8063. doi: 10.3390/ijms22158063. Int J Mol Sci. 2021. PMID: 34360826 Free PMC article.
References
-
- Agis H, Fureder W, Bankl HC, Kundi M, Sperr WR, Willheim M, Boltz-Nitulescu G, Butterfield JH, Kishi K, Lechner K, et al. Comparative immunophenotypic analysis of human mast cells, blood basophils and monocytes. Immunology. 1996;87:535–543. doi:10.1046/j.1365-2567.1996.493578.x. - DOI - PMC - PubMed
-
- Albersheim P, Nevins DJ, English PD, Karr A. A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr Res. 1967;5:340–345. doi:10.1016/S0008-6215(00)80510-8. - DOI
-
- Avril T, North SJ, Haslam SM, Willison HJ, Crocker PR. Probing the cis interactions of the inhibitory receptor Siglec-7 with α2,8-disialylated ligands on natural killer cells and other leukocytes using glycan-specific antibodies and by analysis of α2,8-sialyltransferase gene expression. J Leukoc Biol. 2006;80:787–796. doi:10.1189/jlb.1005559. - DOI - PubMed
-
- Babu P, North SJ, Jang-Lee J, Chalabi S, Mackerness K, Stowell SR, Cummings RD, Rankin S, Dell A, Haslam SM. Structural characterisation of neutrophil glycans by ultra sensitive mass spectrometric glycomics methodology. Glycoconj J. 2009;26:975–986. doi:10.1007/s10719-008-9146-4. - DOI - PMC - PubMed
-
- Bax M, Garcia-Vallejo JJ, Jang-Lee J, North SJ, Gilmartin TJ, Hernandez G, Crocker PR, Leffler H, Head SR, Haslam SM, et al. Dendritic cell maturation results in pronounced changes in glycan expression affecting recognition by siglecs and galectins. J Immunol. 2007;179:8216–8224. - PubMed

