Owl's behavior and neural representation predicted by Bayesian inference
- PMID: 21725311
- PMCID: PMC3145020
- DOI: 10.1038/nn.2872
Owl's behavior and neural representation predicted by Bayesian inference
Abstract
The owl captures prey using sound localization. In the classical model, the owl infers sound direction from the position of greatest activity in a brain map of auditory space. However, this model fails to describe the actual behavior. Although owls accurately localize sources near the center of gaze, they systematically underestimate peripheral source directions. We found that this behavior is predicted by statistical inference, formulated as a Bayesian model that emphasizes central directions. We propose that there is a bias in the neural coding of auditory space, which, at the expense of inducing errors in the periphery, achieves high behavioral accuracy at the ethologically relevant range. We found that the owl's map of auditory space decoded by a population vector is consistent with the behavioral model. Thus, a probabilistic model describes both how the map of auditory space supports behavior and why this representation is optimal.
Figures
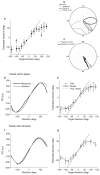
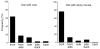


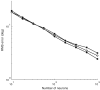

Comment in
-
Prior and prejudice.Nat Neurosci. 2011 Jul 26;14(8):943-5. doi: 10.1038/nn.2883. Nat Neurosci. 2011. PMID: 21792188 No abstract available.
References
-
- Knudsen EI, Blasdel GG, Konishi M. Sound localization by the barn owl (Tyto alba) measured with the search coil technique. J Comp Physiol. 1979;133:1–11.
-
- Jay MF, Sparks DL. Localization of auditory and visual targets for the initiation of saccadic eye movements. In: Berkley MA, Stebbins WC, editors. Comparative perception Basic mechanisms. Wiley, New York; New York, USA: 1990. pp. 351–374.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

