Regulated ATF5 loss-of-function in adult mice blocks formation and causes regression/eradication of gliomas
- PMID: 21725368
- PMCID: PMC3277917
- DOI: 10.1038/onc.2011.276
Regulated ATF5 loss-of-function in adult mice blocks formation and causes regression/eradication of gliomas
Abstract
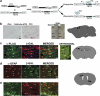
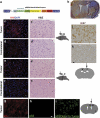
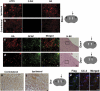
Glioblastomas are among the most incurable cancers. Our past findings indicated that glioblastoma cells, but not neurons or glia, require the transcription factor ATF5 (activating transcription factor 5) for survival. However, it was unknown whether interference with ATF5 function can prevent or promote regression/eradication of malignant gliomas in vivo. To address this issue, we created a mouse model by crossing a human glial fibrillary acidic protein (GFAP) promoter-tetracycline transactivator mouse line with tetracycline operon-dominant negative-ATF5 (d/n-ATF5) mice to establish bi-transgenic mice. In this model, d/n-ATF5 expression is controlled by doxycycline and the promoter for GFAP, a marker for stem/progenitor cells as well as gliomas. Endogenous gliomas were produced with high efficiency by retroviral delivery of platelet-derived growth factor (PDGF)-B and p53-short hairpin RNA (shRNA) in adult bi-transgenic mice in which expression of d/n-ATF5 was spatially and temporally regulated. Induction of d/n-ATF5 before delivery of PDGF-B/p53-shRNA virus greatly reduced the proportion of mice that formed tumors. Moreover, d/n-ATF5 induction after tumor formation led to regression/eradication of detectable gliomas without evident damage to normal brain cells in all 24 mice assessed.
Figures


References
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
-
- Angelastro JM, Canoll PD, Kuo J, Weicker M, Costa A, Bruce JN, et al. Selective destruction of glioblastoma cells by interference with the activity or expression of ATF5. Oncogene. 2006;25:907–916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

