Neoadjuvant chemotherapy is associated with improved survival compared with adjuvant chemotherapy in patients with triple-negative breast cancer only after complete pathologic response
- PMID: 21725686
- PMCID: PMC3892697
- DOI: 10.1245/s10434-011-1877-y
Neoadjuvant chemotherapy is associated with improved survival compared with adjuvant chemotherapy in patients with triple-negative breast cancer only after complete pathologic response
Abstract
Introduction: Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer that is known to be chemosensitive. In patients with TNBC, we sought to compare survival outcomes between patients receiving neoadjuvant chemotherapy, with and without complete pathologic response (pCR), and those receiving adjuvant chemotherapy.
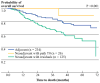
Methods: We performed a retrospective chart review and identified 385 patients with stage I-III TNBC who were treated with neoadjuvant or adjuvant chemotherapy between 2000 and 2008. Patients were divided according to receipt of neoadjuvant chemotherapy with pCR, neoadjuvant chemotherapy without pCR, and adjuvant chemotherapy. Data were compared using Fisher's exact test and analysis of variance (ANOVA). Kaplan-Meier curves were generated.
Results: Of 385 patients, 151 (39%) received neoadjuvant chemotherapy and 234 (61%) received adjuvant chemotherapy. Twenty-six (17%) of those patients receiving neoadjuvant chemotherapy had pCR. After controlling for covariates associated with survival in unadjusted tests, patients undergoing neoadjuvant chemotherapy with residual tumor had significantly worse survival compared with patients receiving adjuvant therapy [hazard ratio (HR) = 0.51, P = 0.007] and a trend towards worse survival compared with patients receiving neoadjuvant therapy with pCR (HR = 0.19, P = 0.10).
Conclusions: Although previous clinical trials have not demonstrated a survival difference between patients receiving neoadjuvant versus adjuvant chemotherapy for breast cancer, our study suggests an overall survival benefit in patients with pCR following neoadjuvant chemotherapy compared with patients receiving adjuvant therapy. It is clear that a prospective study needs to be carried out to better elucidate the timing of chemotherapy in patients with TNBC.
Figures
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–49. - PubMed
-
- Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–18. - PubMed
-
- Rakha E, El-Sayed M, Green A, et al. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical