Differential outcomes in prediabetic vs. overtly diabetic NOD mice nonmyeloablatively conditioned with costimulatory blockade
- PMID: 21726515
- PMCID: PMC3176996
- DOI: 10.1016/j.exphem.2011.06.008
Differential outcomes in prediabetic vs. overtly diabetic NOD mice nonmyeloablatively conditioned with costimulatory blockade
Abstract
Objective: Autoimmune diabetes can be reversed with mixed chimerism. However, the myelotoxic agents currently required to establish chimerism have prevented the translation of this approach to the clinic. Here, we investigated whether multimodal costimulatory blockade would enhance chimerism and promote islet allograft tolerance in spontaneously diabetic nonobese diabetic (NOD) mice.
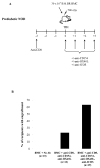
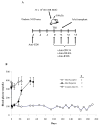
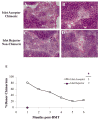
Materials and methods: Prediabetic and spontaneously diabetic NOD mice were preconditioned with anti-CD8 monoclonal antibody before conditioning with 500 cGy total body irradiation and transplantation with 30 × 10(6) B10.BR bone marrow cells. Overtly diabetic animals were conditioned similarly and transplanted with 300 to 400 B10.BR islets. After irradiation, both groups of recipients were treated with anti-CD154, anti-OX40L, and anti-inducible T-cell costimulatory monoclonal antibodies. Urine, blood glucose levels, and chimerism were monitored.
Results: Conditioning of NOD mice with costimulatory blockade significantly enhanced engraftment, with 61% of mice engrafting at 1 month. Eleven of 12 chimeric animals with engraftment at 1 month remained diabetes-free over a 12-month follow-up, whereas nonchimeric animals progressed to diabetes. In contrast, similar conditioning prolonged islet allograft survival in only 2 of 11 overtly diabetic NOD recipients. Chimerism levels in the 9 islet rejector animals were 0%.
Conclusions: Although nonmyeloablative conditioning reversed the autoimmune process in prediabetic NOD mice, the same regimen was significantly less effective in establishing chimerism and reversing autoimmune diabetes in spontaneously diabetic NOD mice.
Copyright © 2011 ISEH - Society for Hematology and Stem Cells. Published by Elsevier Inc. All rights reserved.
Figures


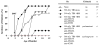



Similar articles
-
Distinct requirements for achievement of allotolerance versus reversal of autoimmunity via nonmyeloablative mixed chimerism induction in NOD mice.Transplantation. 2010 Jan 15;89(1):23-32. doi: 10.1097/TP.0b013e3181c4692e. Transplantation. 2010. PMID: 20061915 Free PMC article.
-
Preconditioning of NOD mice with anti-CD8 mAb and costimulatory blockade enhances chimerism and tolerance and prevents diabetes, while depletion of alpha beta-TCR+ and CD4+ cells negates the effect.Blood. 2005 Mar 15;105(6):2577-84. doi: 10.1182/blood-2004-04-1340. Epub 2004 Oct 21. Blood. 2005. PMID: 15498851
-
A substantial level of donor hematopoietic chimerism is required to protect donor-specific islet grafts in diabetic NOD mice.Transplantation. 2003 Apr 15;75(7):909-15. doi: 10.1097/01.TP.0000057832.92231.F5. Transplantation. 2003. PMID: 12698073
-
Islet allograft rejection in nonobese diabetic mice involves the common gamma-chain and CD28/CD154-dependent and -independent mechanisms.J Immunol. 2003 Oct 1;171(7):3878-85. doi: 10.4049/jimmunol.171.7.3878. J Immunol. 2003. PMID: 14500690
-
Recovery of the endogenous beta cell function in the NOD model of autoimmune diabetes.Stem Cells. 2003;21(4):377-88. doi: 10.1634/stemcells.21-4-377. Stem Cells. 2003. PMID: 12832692 Review.
Cited by
-
Stability of Chimerism in Non-Obese Diabetic Mice Achieved By Rapid T Cell Depletion Is Associated With High Levels of Donor Cells Very Early After Transplant.Front Immunol. 2018 Apr 24;9:837. doi: 10.3389/fimmu.2018.00837. eCollection 2018. Front Immunol. 2018. PMID: 29740442 Free PMC article.
References
-
- Nelson JL, Torrez R, Louie FM, Choe OS, Storb R, Sullivan KM. Pre-existing Autoimmune Disease in Patients with Longterm Survival after Allogeneic Bone Marrow Transplantation. J Rheumatol. 1997;24:23–29. - PubMed
-
- Ildstad ST, Chilton PM, Xu H, Domenick MA, Ray MB. Preconditioning of NOD mice with anti-CD8 mAb and co-stimulatory blockade enhances chimerism and tolerance and prevents diabetes while depletion of {alpha}{beta}-TCR+ and CD4+ cells negates the effect. Blood. 2005;105:2577–2584. - PubMed
-
- Burt RK, Oyama Y, Verda L, et al. Induction of remission of severe and refractory rheumatoid arthritis by allogeneic mixed chimerism. Arthritis Rheum. 2004;50:2466–2470. - PubMed
-
- Van WB, Sprangers B, Rutgeerts O, et al. Allogeneic bone marrow transplantation in models of experimental autoimmune encephalomyelitis: evidence for a graft-versus-autoimmunity effect. Biol Blood Marrow Transplant. 2007;13:627–637. - PubMed
-
- Li H, Kaufman CL, Boggs SS, Johnson PC, Patrene KD, Ildstad ST. Mixed allogeneic chimerism induced by a sublethal approach prevents autoimmune diabetes and reverses insulitis in non-obese diabetic (NOD) mice. J Immunol. 1996;156:380–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

