Shroom3 and a Pitx2-N-cadherin pathway function cooperatively to generate asymmetric cell shape changes during gut morphogenesis
- PMID: 21726547
- PMCID: PMC3619216
- DOI: 10.1016/j.ydbio.2011.06.027
Shroom3 and a Pitx2-N-cadherin pathway function cooperatively to generate asymmetric cell shape changes during gut morphogenesis
Abstract
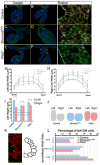
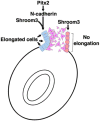
The cytoskeletal protein Shroom3 is a potent inducer of epithelial cell shape change and is required for lens and neural plate morphogenesis. Analysis of gut morphogenesis in Shroom3 deficient mouse embryos revealed that the direction of gut rotation is also disrupted. It was recently established that Pitx2-dependent, asymmetrical cellular behaviors in the dorsal mesentery (DM) of the early mid-gut, a structure connecting the gut-tube to the rest of the embryo, contribute to the direction of gut rotation in chicken embryos by influencing the direction of the dorsal mesenteric tilt. Asymmetric cell shapes in the DM epithelium are hypothesized to contribute to the tilt, however, it is unclear what lies downstream of Pitx2 to alter epithelial cell shape. The cells of the left DM epithelium in either Pitx2 or Shroom3 deficient embryos are shorter and wider than those in control embryos and resemble the shape of those on the right, demonstrating that like Pitx2, Shroom3 is required for cell shape asymmetry and the leftward DM tilt. Because N-cadherin expression is specific to the left side and is Pitx2 dependent, we determined whether Shroom3 and N-cadherin function together to regulate cell shape in the left DM epithelium. Analysis of mouse embryos lacking one allele of both Shroom3 and N-cadherin revealed that they possess shorter and wider left epithelial DM cells when compared with Shroom3 or N-cadherin heterozygous embryos. This indicates a genetic interaction. Together these data provide evidence that Shroom3 and N-cadherin function cooperatively downstream of Pitx2 to directly regulate cell shape changes necessary for early gut tube morphogenesis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Campione M, Steinbeisser H, Schweickert A, Deissler K, van Bebber F, Lowe LA, Nowotschin S, Viebahn C, Haffter P, Kuehn MR, et al. The homeobox gene Pitx2: mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development. 1999;126(6):1225–34. - PubMed
-
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126(20):4643–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

