Two-step aminoacylation of tRNA without channeling in Archaea
- PMID: 21726564
- PMCID: PMC3156345
- DOI: 10.1016/j.jmb.2011.06.039
Two-step aminoacylation of tRNA without channeling in Archaea
Erratum in
- J Mol Biol. 2011 Oct 14;413(1):292-3
Abstract
Catalysis of sequential reactions is often envisaged to occur by channeling of substrate between enzyme active sites without release into bulk solvent. However, while there are compelling physiological rationales for direct substrate transfer, proper experimental support for the hypothesis is often lacking, particularly for metabolic pathways involving RNA. Here, we apply transient kinetics approaches developed to study channeling in bienzyme complexes to an archaeal protein synthesis pathway featuring the misaminoacylated tRNA intermediate Glu-tRNA(Gln). Experimental and computational elucidation of a kinetic and thermodynamic framework for two-step cognate Gln-tRNA(Gln) synthesis demonstrates that the misacylating aminoacyl-tRNA synthetase (GluRS(ND)) and the tRNA-dependent amidotransferase (GatDE) function sequentially without channeling. Instead, rapid processing of the misacylated tRNA intermediate by GatDE and preferential elongation factor binding to the cognate Gln-tRNA(Gln) together permit accurate protein synthesis without formation of a binary protein-protein complex between GluRS(ND) and GatDE. These findings establish an alternate paradigm for protein quality control via two-step pathways for cognate aminoacyl-tRNA formation.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

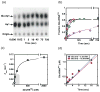



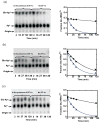


References
-
- Anderson KS. Fundamental mechanisms of substrate channeling. Methods Enzymol. 1999;308:111–126. - PubMed
-
- Huang X, Holden HM, Raushel FM. Channeling of substrates and intermediates in enzyme-catalyzed reactions. Annu Rev Biochem. 2001;70:149–180. - PubMed
-
- An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320:103–106. - PubMed
-
- Lenaz G, Genova ML. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxid Redox Signal. 2010;12:961–1008. - PubMed
-
- Meyer FM, Gerwig J, Hammer E, Herzberg C, Commichau FM, Volker U, Stulke J. Physical interactions between tricarboxylic acid cycle enzymes in Bacillus subtilis: evidence for a metabolon. Metab Eng. 2011;13:18–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

