All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells
- PMID: 21726834
- PMCID: PMC4103692
- DOI: 10.1016/j.stem.2011.04.021
All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells
Abstract
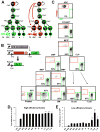
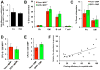
While it is clear that a single hematopoietic stem cell (HSC) is capable of giving rise to all other hematopoietic cell types, the differentiation paths beyond HSC remain controversial. Contradictory reports on the lineage potential of progenitor populations have questioned their physiological contribution of progenitor populations to multilineage differentiation. Here, we established a lineage tracing mouse model that enabled direct assessment of differentiation pathways in vivo. We provide definitive evidence that differentiation into all hematopoietic lineages, including megakaryocyte/erythroid cell types, involves Flk2-expressing non-self-renewing progenitors. A Flk2+ stage was used during steady-state hematopoiesis, after irradiation-induced stress and upon HSC transplantation. In contrast, HSC origin and maintenance do not include a Flk2+ stage. These data demonstrate that HSC specification and maintenance are Flk2 independent, and that hematopoietic lineage separation occurs downstream of Flk2 upregulation.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. - PubMed
-
- Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. - PubMed
-
- Akashi K. Lymphoid lineage fate decision of hematopoietic stem cells. Ann N Y Acad Sci. 2009;1176:18–25. - PubMed
-
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
-
- Akashi K, Traver D, Zon LI. The complex cartography of stem cell commitment. Cell. 2005;121:160–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

