L-3-Phosphoserine phosphatase (PSPH) regulates cutaneous squamous cell carcinoma proliferation independent of L-serine biosynthesis
- PMID: 21726982
- PMCID: PMC3152677
- DOI: 10.1016/j.jdermsci.2011.06.001
L-3-Phosphoserine phosphatase (PSPH) regulates cutaneous squamous cell carcinoma proliferation independent of L-serine biosynthesis
Abstract
Background: L-3-Phosphoserine phosphatase (PSPH) is a highly conserved and widely expressed member of the haloacid dehalogenase superfamily and the rate-limiting enzyme in l-serine biosynthesis. We previously found Psph expression to be uniquely upregulated in a α6β4 integrin transgenic mouse model that is predisposed to epidermal hyperproliferation and squamous cell carcinoma (SCC) formation implicating a role for Psph in epidermal homeostasis.
Objective: We examined the status of PSPH in normal skin epidermis and skin tumors along with its sub-cellular localization in epidermal keratinocytes and its requirement for squamous cell carcinoma (SCC) proliferation.
Methods: First, an immunohistochemical study was performed for PSPH in normal skin and skin cancer specimens and in cultured keratinocytes. Next, biochemical analyses were performed to confirm localization of PSPH and to identify candidate binding proteins. Finally, proliferation and apoptosis studies were performed in human SCC and normal keratinocytes, respectively, transduced with vectors encoding small hairpin RNAs targeting PSPH or overexpressing a phosphatase-deficient PSPH mutant.
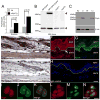
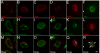
Results: PSPH is expressed throughout the proliferative layer of the epidermis and hair follicles in rodent and human skin and is highly induced in SCC. In keratinocytes, PSPH is a cytoplasmic protein that primarily localizes to endosomes and is present primarily as a homodimer. Knock down of PSPH dramatically diminished SCC cell proliferation and cyclin D1 levels in the presence of exogenous of l-serine production suggesting a non-canonical role for PSPH in epithelial carcinogenesis.
Conclusions: Psph is highly induced in proliferative normal keratinocytes and in skin tumors. PSPH appears to be critical for the proliferation of SCC cells; however, this phenomenon may not involve the phosphoserine metabolic pathway.
Copyright © 2011 Japanese Society for Investigative Dermatology. Published by Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
The authors report no conflict of interest.
Figures



References
-
- Borkenhagen LF, Kennedy EP. The enzymic equilibration of L-serine with O-phospho-L-serine. Biochim Biophys Acta. 1958;28:222–223. - PubMed
-
- Neuhaus FC, Byrne WL. O-Phosphoserine phosphatase. Biochim Biophys Acta. 1958;28:223–224. - PubMed
-
- Collet JF, Gerin I, Rider MH, Veiga-da-Cunha M, Van Schaftingen E. Human L-3-phosphoserine phosphatase: sequence, expression and evidence for a phosphoenzyme intermediate. FEBS Lett. 1997;411:251–254. - PubMed
-
- Collet JF, Stroobant V, Pirard M, Delpierre G, Van Schaftingen E. A new class of phosphotransferases phosphorylated on an aspartate residue in an amino-terminal DXDX(T/V) motif. J Biol Chem. 1998;273:14107–14112. - PubMed
-
- Collet JF, Stroobant V, Van Schaftingen E. Mechanistic studies of phosphoserine phosphatase, an enzyme related to P-type ATPases. J Biol Chem. 1999;274:33985–33990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

