The immunoreceptor adapter protein DAP12 suppresses B lymphocyte-driven adaptive immune responses
- PMID: 21727189
- PMCID: PMC3149228
- DOI: 10.1084/jem.20101623
The immunoreceptor adapter protein DAP12 suppresses B lymphocyte-driven adaptive immune responses
Abstract
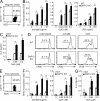
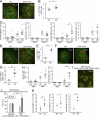
DAP12, an immunoreceptor tyrosine-based activation motif-bearing adapter protein, is involved in innate immunity mediated by natural killer cells and myeloid cells. We show that DAP12-deficient mouse B cells and B cells from a patient with Nasu-Hakola disease, a recessive genetic disorder resulting from loss of DAP12, showed enhanced proliferation after stimulation with anti-IgM or CpG. Myeloid-associated immunoglobulin-like receptor (MAIR) II (Cd300d) is a DAP12-associated immune receptor. Like DAP12-deficient B cells, MAIR-II-deficient B cells were hyperresponsive. Expression of a chimeric receptor composed of the MAIR-II extracellular domain directly coupled to DAP12 into the DAP12-deficient or MAIR-II-deficient B cells suppressed B cell receptor (BCR)-mediated proliferation. The chimeric MAIR-II-DAP12 receptor recruited the SH2 domain-containing protein tyrosine phosphatase 1 (SHP-1) after BCR stimulation. DAP12-deficient mice showed elevated serum antibodies against self-antigens and enhanced humoral immune responses against T cell-dependent and T cell-independent antigens. Thus, DAP12-coupled MAIR-II negatively regulates B cell-mediated adaptive immune responses.
Figures
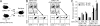

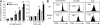


References
-
- Allman D., Lindsley R.C., DeMuth W., Rudd K., Shinton S.A., Hardy R.R. 2001. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 167:6834–6840 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

