Applying the brakes to multisite SR protein phosphorylation: substrate-induced effects on the splicing kinase SRPK1
- PMID: 21728354
- PMCID: PMC3153611
- DOI: 10.1021/bi2007993
Applying the brakes to multisite SR protein phosphorylation: substrate-induced effects on the splicing kinase SRPK1
Abstract
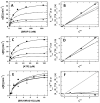
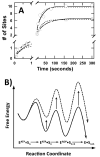
To investigate how a protein kinase interacts with its protein substrate during extended, multisite phosphorylation, the kinetic mechanism of a protein kinase involved in mRNA splicing control was investigated using rapid quench flow techniques. The protein kinase SRPK1 phosphorylates ~10 serines in the arginine--serine-rich domain (RS domain) of the SR protein SRSF1 in a C- to N-terminal direction, a modification that directs this essential splicing factor from the cytoplasm to the nucleus. Transient-state kinetic experiments illustrate that the first phosphate is added rapidly onto the RS domain of SRSF1 (t(1/2) = 0.1 s) followed by slower, multisite phosphorylation at the remaining serines (t(1/2) = 15 s). Mutagenesis experiments suggest that efficient phosphorylation rates are maintained by an extensive hydrogen bonding and electrostatic network between the RS domain of the SR protein and the active site and docking groove of the kinase. Catalytic trapping and viscosometric experiments demonstrate that while the phosphoryl transfer step is fast, ADP release limits multisite phosphorylation. By studying phosphate incorporation into selectively pre-phosphorylated forms of the enzyme-substrate complex, the kinetic mechanism for site-specific phosphorylation along the reaction coordinate was assessed. The binding affinity of the SR protein, the phosphoryl transfer rate, and ADP exchange rate were found to decline significantly as a function of progressive phosphorylation in the RS domain. These findings indicate that the protein substrate actively modulates initiation, extension, and termination events associated with prolonged, multisite phosphorylation.
Figures





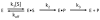
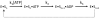
References
-
- Jurica MS, Moore MJ. Pre-mRNA splicing: awash in a sea of proteins. Mol Cell. 2003;12:5–14. - PubMed
-
- Blencowe BJ, Bowman JA, McCracken S, Rosonina E. SR-related proteins and the processing of messenger RNA precursors. Biochem Cell Biol. 1999;77:277–291. - PubMed
-
- Gui JF, Lane WS, Fu XD. A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature. 1994;369:678–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

