The receptors for gibbon ape leukemia virus and amphotropic murine leukemia virus are not downregulated in productively infected cells
- PMID: 21729311
- PMCID: PMC3136417
- DOI: 10.1186/1742-4690-8-53
The receptors for gibbon ape leukemia virus and amphotropic murine leukemia virus are not downregulated in productively infected cells
Abstract
Background: Over the last several decades it has been noted, using a variety of different methods, that cells infected by a specific gammaretrovirus are resistant to infection by other retroviruses that employ the same receptor; a phenomenon termed receptor interference. Receptor masking is thought to provide an earlier means of blocking superinfection, whereas receptor down regulation is generally considered to occur in chronically infected cells.
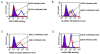
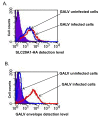
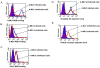
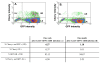
Results: We used replication-competent GFP-expressing viruses containing either an amphotropic murine leukemia virus (A-MLV) or the gibbon ape leukemia virus (GALV) envelope. We also constructed similar viruses containing fluorescence-labeled Gag proteins for the detection of viral particles. Using this repertoire of reagents together with a wide range of antibodies, we were able to determine the presence and availability of viral receptors, and detect viral envelope proteins and particles presence on the cell surface of chronically infected cells.
Conclusions: A-MLV or GALV receptors remain on the surface of chronically infected cells and are detectable by respective antibodies, indicating that these receptors are not downregulated in these infected cells as previously proposed. We were also able to detect viral envelope proteins on the infected cell surface and infected cells are unable to bind soluble A-MLV or GALV envelopes indicating that receptor binding sites are masked by endogenously expressed A-MLV or GALV viral envelope. However, receptor masking does not completely prevent A-MLV or GALV superinfection.
Figures




Similar articles
-
Gibbon ape leukemia virus and the amphotropic murine leukemia virus 4070A exhibit an unusual interference pattern on E36 Chinese hamster cells.J Virol. 1993 Sep;67(9):5472-7. doi: 10.1128/JVI.67.9.5472-5477.1993. J Virol. 1993. PMID: 8394458 Free PMC article.
-
Primate gammaretroviruses require an ancillary factor not required for murine gammaretroviruses to infect BHK cells.J Virol. 2011 Apr;85(7):3498-506. doi: 10.1128/JVI.02586-10. Epub 2011 Jan 26. J Virol. 2011. PMID: 21270153 Free PMC article.
-
Cellular and species resistance to murine amphotropic, gibbon ape, and feline subgroup C leukemia viruses is strongly influenced by receptor expression levels and by receptor masking mechanisms.J Virol. 2000 Oct;74(20):9797-801. doi: 10.1128/jvi.74.20.9797-9801.2000. J Virol. 2000. PMID: 11000257 Free PMC article.
-
A new look at the origins of gibbon ape leukemia virus.Virus Genes. 2017 Apr;53(2):165-172. doi: 10.1007/s11262-017-1436-0. Epub 2017 Feb 20. Virus Genes. 2017. PMID: 28220345 Review.
-
Xenotropism: the elusive viral receptor finally uncovered.Proc Natl Acad Sci U S A. 1999 Feb 2;96(3):802-4. doi: 10.1073/pnas.96.3.802. Proc Natl Acad Sci U S A. 1999. PMID: 9927648 Free PMC article. Review. No abstract available.
Cited by
-
Efficient tumor transduction and antitumor efficacy in experimental human osteosarcoma using retroviral replicating vectors.Cancer Gene Ther. 2019 Feb;26(1-2):41-47. doi: 10.1038/s41417-018-0037-y. Epub 2018 Jul 25. Cancer Gene Ther. 2019. PMID: 30042500 Free PMC article.
-
Retroviral Replicating Vectors Mediated Prodrug Activator Gene Therapy in a Gastric Cancer Model.Int J Mol Sci. 2023 Oct 2;24(19):14823. doi: 10.3390/ijms241914823. Int J Mol Sci. 2023. PMID: 37834271 Free PMC article.
-
A mutant retroviral receptor restricts virus superinfection interference and productive infection.Retrovirology. 2012 Jun 12;9:51. doi: 10.1186/1742-4690-9-51. Retrovirology. 2012. PMID: 22691439 Free PMC article.
-
Dual-vector prodrug activator gene therapy using retroviral replicating vectors.Cancer Gene Ther. 2019 May;26(5-6):128-135. doi: 10.1038/s41417-018-0051-0. Epub 2018 Oct 22. Cancer Gene Ther. 2019. PMID: 30348946 Free PMC article.
-
Unique Structure and Distinctive Properties of the Ancient and Ubiquitous Gamma-Type Envelope Glycoprotein.Viruses. 2023 Jan 18;15(2):274. doi: 10.3390/v15020274. Viruses. 2023. PMID: 36851488 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

