Structural and functional basis for RNA cleavage by Ire1
- PMID: 21729333
- PMCID: PMC3149027
- DOI: 10.1186/1741-7007-9-47
Structural and functional basis for RNA cleavage by Ire1
Abstract
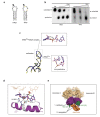
Background: The unfolded protein response (UPR) controls the protein folding capacity of the endoplasmic reticulum (ER). Central to this signaling pathway is the ER-resident bifunctional transmembrane kinase/endoribonuclease Ire1. The endoribonuclease (RNase) domain of Ire1 initiates a non-conventional mRNA splicing reaction, leading to the production of a transcription factor that controls UPR target genes. The mRNA splicing reaction is an obligatory step of Ire1 signaling, yet its mechanism has remained poorly understood due to the absence of substrate-bound crystal structures of Ire1, the lack of structural similarity between Ire1 and other RNases, and a scarcity of quantitative enzymological data. Here, we experimentally define the active site of Ire1 RNase and quantitatively evaluate the contribution of the key active site residues to catalysis.
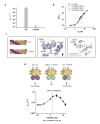
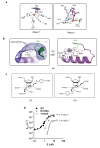
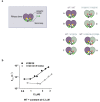
Results: This analysis and two new crystal structures suggest that Ire1 RNase uses histidine H1061 and tyrosine Y1043 as the general acid-general base pair contributing ≥7.6 kcal/mol and 1.4 kcal/mol to transition state stabilization, respectively, and asparagine N1057 and arginine R1056 for coordination of the scissile phosphate. Investigation of the stem-loop recognition revealed that additionally to the stem-loops derived from the classic Ire1 substrates HAC1 and Xbp1 mRNA, Ire1 can site-specifically and rapidly cleave anticodon stem-loop (ASL) of unmodified tRNAPhe, extending known substrate specificity of Ire1 RNase.
Conclusions: Our data define the catalytic center of Ire1 RNase and suggest a mechanism of RNA cleavage: each RNase monomer apparently contains a separate catalytic apparatus for RNA cleavage, whereas two RNase subunits contribute to RNA stem-loop docking. Conservation of the key residues among Ire1 homologues suggests that the mechanism elucidated here for yeast Ire1 applies to Ire1 in metazoan cells, and to the only known Ire1 homologue RNase L.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

