Polarization sensitive optical coherence tomography in the human eye
- PMID: 21729763
- PMCID: PMC3205186
- DOI: 10.1016/j.preteyeres.2011.06.003
Polarization sensitive optical coherence tomography in the human eye
Abstract
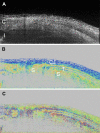
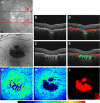
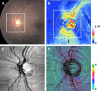
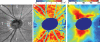
Optical coherence tomography (OCT) has become a well established imaging tool in ophthalmology. The unprecedented depth resolution that is provided by this technique yields valuable information on different ocular tissues ranging from the anterior to the posterior eye segment. Polarization sensitive OCT (PS-OCT) extends the concept of OCT and utilizes the information that is carried by polarized light to obtain additional information on the tissue. Several structures in the eye (e.g. cornea, retinal nerve fiber layer, retinal pigment epithelium) alter the polarization state of the light and show therefore a tissue specific contrast in PS-OCT images. First this review outlines the basic concepts of polarization changing light-tissue interactions and gives a short introduction in PS-OCT instruments for ophthalmic imaging. In a second part a variety of different applications of this technique are presented in ocular imaging that are ranging from the anterior to the posterior eye segment. Finally the benefits of the method for imaging different diseases as, e.g., age related macula degeneration (AMD) or glaucoma is demonstrated.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures














References
-
- Ahlers C., Gotzinger E., Pircher M., Golbaz I., Prager F., Schutze C., Baumann B., Hitzenberger C.K., Schmidt-Erfurth U. Imaging of the retinal pigment epithelium in age-related macular degeneration using polarization-sensitive optical coherence tomography. Investigative Ophthalmology & Visual Science. 2010;51:2149–2157. - PMC - PubMed
-
- Ahlers C., Simader C., Geitzenauer W., Stock G., Stetson P., Dastmalchi S., Schmidt-Erfurth U. Automatic segmentation in three-dimensional analysis of fibrovascular pigmentepithelial detachment using high-definition optical coherence tomography. British Journal of Ophthalmology. 2008;92:197–203. - PubMed
-
- Anand R., Bressler S.B., Davis M.D., Ferris F.L., Klein R., Lindblad A.S., Milton R.C., Sperduto R.D., Grp, A.R.E.D.S.R Risk factors associated with age-related macular degeneration – A case-control study in the age-related eye disease study: age-Related Eye Disease Study report number 3. Ophthalmology. 2000;107:2224–2232. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

