Stepwise insertion and inversion of a type II signal anchor sequence in the ribosome-Sec61 translocon complex
- PMID: 21729785
- PMCID: PMC3181170
- DOI: 10.1016/j.cell.2011.06.004
Stepwise insertion and inversion of a type II signal anchor sequence in the ribosome-Sec61 translocon complex
Abstract
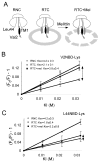
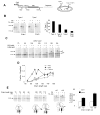
In eukaryotic cells, the ribosome-Sec61 translocon complex (RTC) establishes membrane protein topology by cotranslationally partitioning nascent polypeptides into the cytosol, ER lumen, and lipid bilayer. Using photocrosslinking, collisional quenching, cysteine accessibility, and protease protection, we show that a canonical type II signal anchor (SA) acquires its topology through four tightly coupled and mechanistically distinct steps: (1) head-first insertion into Sec61α, (2) nascent chain accumulation within the RTC, (3) inversion from type I to type II topology, and (4) stable translocation of C-terminal flanking residues. Progression through each stage is induced by incremental increases in chain length and involves abrupt changes in the molecular environment of the SA. Importantly, type II SA inversion deviates from a type I SA at an unstable intermediate whose topology is controlled by dynamic interactions between the ribosome and translocon. Thus, the RTC coordinates SA topogenesis within a protected environment via sequential energetic transitions of the TM segment.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

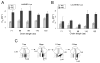



Comment in
-
A flip turn for membrane protein insertion.Cell. 2011 Jul 8;146(1):13-5. doi: 10.1016/j.cell.2011.06.028. Cell. 2011. PMID: 21729778 Free PMC article.
Similar articles
-
The Ribosome-Sec61 Translocon Complex Forms a Cytosolically Restricted Environment for Early Polytopic Membrane Protein Folding.J Biol Chem. 2015 Nov 27;290(48):28944-52. doi: 10.1074/jbc.M115.672261. Epub 2015 Aug 7. J Biol Chem. 2015. PMID: 26254469 Free PMC article.
-
Sequence-specific retention and regulated integration of a nascent membrane protein by the endoplasmic reticulum Sec61 translocon.Mol Biol Cell. 2009 Jan;20(2):685-98. doi: 10.1091/mbc.e08-09-0902. Epub 2008 Nov 19. Mol Biol Cell. 2009. PMID: 19019984 Free PMC article.
-
Role of Sec61alpha in the regulated transfer of the ribosome-nascent chain complex from the signal recognition particle to the translocation channel.Cell. 2000 Feb 4;100(3):333-43. doi: 10.1016/s0092-8674(00)80669-8. Cell. 2000. PMID: 10676815
-
Functions and Mechanisms of the Human Ribosome-Translocon Complex.Subcell Biochem. 2019;93:83-141. doi: 10.1007/978-3-030-28151-9_4. Subcell Biochem. 2019. PMID: 31939150 Review.
-
Topogenesis of membrane proteins at the endoplasmic reticulum.Biochemistry. 2004 Oct 12;43(40):12716-22. doi: 10.1021/bi048368m. Biochemistry. 2004. PMID: 15461443 Review.
Cited by
-
CFTR trafficking mutations disrupt cotranslational protein folding by targeting biosynthetic intermediates.Nat Commun. 2020 Aug 26;11(1):4258. doi: 10.1038/s41467-020-18101-8. Nat Commun. 2020. PMID: 32848127 Free PMC article.
-
Exploring the nature of the translocon-assisted protein insertion.Proc Natl Acad Sci U S A. 2013 Jan 8;110(2):495-500. doi: 10.1073/pnas.1220361110. Epub 2012 Dec 26. Proc Natl Acad Sci U S A. 2013. PMID: 23269832 Free PMC article.
-
Cotranslational stabilization of Sec62/63 within the ER Sec61 translocon is controlled by distinct substrate-driven translocation events.Mol Cell. 2015 Apr 16;58(2):269-83. doi: 10.1016/j.molcel.2015.02.018. Epub 2015 Mar 19. Mol Cell. 2015. PMID: 25801167 Free PMC article.
-
Membrane Protein Integration and Topogenesis at the ER.Protein J. 2019 Jun;38(3):306-316. doi: 10.1007/s10930-019-09827-6. Protein J. 2019. PMID: 30927129 Review.
-
A Shared Mechanism for the Folding of Voltage-Gated K+ Channels.Biochemistry. 2019 Mar 26;58(12):1660-1671. doi: 10.1021/acs.biochem.9b00068. Epub 2019 Mar 7. Biochemistry. 2019. PMID: 30793887 Free PMC article.
References
-
- Crowley K, Liao S, Worrell V, Reinhart G, Johnson A. Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell. 1994;78:461–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

