Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis
- PMID: 21729786
- PMCID: PMC3244213
- DOI: 10.1016/j.cell.2011.05.040
Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis
Abstract
Mechanotransduction is a key determinant of tissue homeostasis and tumor progression. It is driven by intercellular adhesions, cell contractility, and forces generated within the microenvironment and is dependent on extracellular matrix composition, organization, and compliance. We show that caveolin-1 (Cav1) favors cell elongation in three-dimensional cultures and promotes Rho- and force-dependent contraction, matrix alignment, and microenvironment stiffening through regulation of p190RhoGAP. In turn, microenvironment remodeling by Cav1 fibroblasts forces cell elongation. Cav1-deficient mice have disorganized stromal tissue architecture. Stroma associated with human carcinomas and melanoma metastases is enriched in Cav1-expressing carcinoma-associated fibroblasts (CAFs). Cav1 expression in breast CAFs correlates with low survival, and Cav1 depletion in CAFs decreases CAF contractility. Consistently, fibroblast expression of Cav1, through p190RhoGAP regulation, favors directional migration and invasiveness of carcinoma cells in vitro. In vivo, stromal Cav1 remodels peri- and intratumoral microenvironments to facilitate tumor invasion, correlating with increased metastatic potency. Thus, Cav1 modulates tissue responses through force-dependent architectural regulation of the microenvironment.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

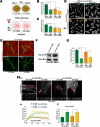
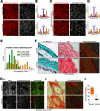
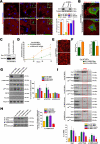
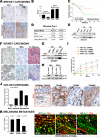

Comment in
-
Microenvironment: partners in crime.Nat Rev Cancer. 2011 Aug 11;11(9):624-5. doi: 10.1038/nrc3123. Nat Rev Cancer. 2011. PMID: 21833027 No abstract available.
-
Replication Study: Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis.Elife. 2019 Dec 17;8:e45120. doi: 10.7554/eLife.45120. Elife. 2019. PMID: 31845647 Free PMC article.
References
-
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annual Review of Cell and Developmental Biology. 2003;19:677–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

