Function of the sex chromosomes in mammalian fertility
- PMID: 21730045
- PMCID: PMC3179336
- DOI: 10.1101/cshperspect.a002675
Function of the sex chromosomes in mammalian fertility
Abstract
The sex chromosomes play a highly specialized role in germ cell development in mammals, being enriched in genes expressed in the testis and ovary. Sex chromosome abnormalities (e.g., Klinefelter [XXY] and Turner [XO] syndrome) constitute the largest class of chromosome abnormalities and the commonest genetic cause of infertility in humans. Understanding how sex-gene expression is regulated is therefore critical to our understanding of human reproduction. Here, we describe how the expression of sex-linked genes varies during germ cell development; in females, the inactive X chromosome is reactivated before meiosis, whereas in males the X and Y chromosomes are inactivated at this stage. We discuss the epigenetics of sex chromosome inactivation and how this process has influenced the gene content of the mammalian X and Y chromosomes. We also present working models for how perturbations in sex chromosome inactivation or reactivation result in subfertility in the major classes of sex chromosome abnormalities.
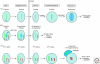
Figures


References
-
- Andina RJ 1978. A study of X chromosome regulation during oogenesis in the mouse. Exp Cell Res 111: 211–218 - PubMed
-
- Ashley T 2000. An integration of old and new perspectives of mammalian meiotic sterility. In Results and problems in cell differentiation, Vol. 2 (ed. McElreavey K), pp. 131–173 Springer-Verlag, Berlin; - PubMed
-
- Baarends WM, Hoogerbrugge JW, Roest HP, Ooms M, Vreeburg J, Hoeijmakers JH, Grootegoed JA 1999. Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev Biol 207: 322–333 - PubMed
-
- Baudat F, de Massy B 2007. Regulating double-stranded DNA break repair towards crossover or non-crossover during mammalian meiosis. Chromosome Res 15: 565–577 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
