Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies
- PMID: 21730149
- PMCID: PMC3145697
- DOI: 10.1073/pnas.1018793108
Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies
Abstract
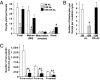
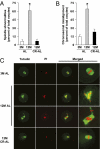
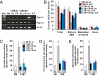
Increased meiotic spindle abnormalities and aneuploidy in oocytes of women of advanced maternal ages lead to elevated rates of infertility, miscarriage, and trisomic conceptions. Despite the significance of the problem, strategies to sustain oocyte quality with age have remained elusive. Here we report that adult female mice maintained under 40% caloric restriction (CR) did not exhibit aging-related increases in oocyte aneuploidy, chromosomal misalignment on the metaphase plate, meiotic spindle abnormalities, or mitochondrial dysfunction (aggregation, impaired ATP production), all of which occurred in oocytes of age-matched ad libitum-fed controls. The effects of CR on oocyte quality in aging females were reproduced by deletion of the metabolic regulator, peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α). Thus, CR during adulthood or loss of PGC-1α function maintains female germline chromosomal stability and its proper segregation during meiosis, such that ovulated oocytes of aged female mice previously maintained on CR or lacking PGC-1α are comparable to those of young females during prime reproductive life.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

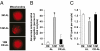
Comment in
-
Re: prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies.J Urol. 2012 May;187(5):1925-6. doi: 10.1016/j.juro.2012.01.042. Epub 2012 Mar 17. J Urol. 2012. PMID: 22494763 No abstract available.
References
-
- Henderson SA, Edwards RG. Chiasma frequency and maternal age in mammals. Nature. 1968;218:22–28. - PubMed
-
- Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70:11–17. - PubMed
-
- Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–2222. - PubMed
-
- Hunt PA, Hassold TJ. Human female meiosis: What makes a good egg go bad? Trends Genet. 2008;24:86–93. - PubMed
-
- Ventura SJ. Trends and variations in first births to older women, United States, 1970-86. Vital Health Stat 21. 1989;47:1–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

