Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy
- PMID: 21730275
- PMCID: PMC3157979
- DOI: 10.1200/JCO.2010.32.2321
Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy
Abstract
Purpose: Trastuzumab resistance has been linked to activation of the phosphoinositol 3-kinase (PI3K) pathway. Phosphatase and tensin homolog (PTEN) is a dual phosphatase that counteracts the PI3K function; PTEN loss leads to activation of the Akt cascade and the downstream mammalian target of rapamycin (mTOR). Preclinical studies demonstrated that mTOR inhibition sensitized the response to trastuzumab in mice with HER2 overexpressing and PTEN-deficient breast xenografts. Our trial evaluated the safety and efficacy of the combination of everolimus and trastuzumab in women with HER2-overexpressing metastatic breast cancer (MBC) that progressed on trastuzumab-based therapy.
Patients and methods: This represents a pooled analysis (n = 47), stemming from two trials that occurred concurrently in The University of Texas MD Anderson Cancer Center, Beth Israel Deaconess Medical Center, and Dana-Farber Cancer Institute. Patients with HER2-overexpressing MBC who had progressed on trastuzumab-based therapy received trastuzumab every 3 weeks in combination with daily everolimus.
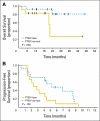
Results: Among 47 patients, the combination of everolimus and trastuzumab provided partial responses in seven patients (15%) and persistent stable disease (lasting 6 months or longer) in nine patients (19%), resulting in a clinical benefit rate of 34%. The median progression-free survival (PFS) was 4.1 month. Fatigue, infection, and mucositis were the predominant nonhematologic toxicities. Trastuzumab did not have significant influence on the pharmacokinetic profile of everolimus. Patients with PTEN loss demonstrated decreased overall survival (P = .048). However, PFS was not affected by PTEN loss.
Conclusion: Inhibition of mTOR results in clinical benefit and disease response in patients with trastuzumab-resistant HER2-overexpressing MBC.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Targeted therapy for human epidermal growth factor receptor 2-positive breast cancer: can there be too many active drugs?J Clin Oncol. 2011 Aug 10;29(23):3111-3. doi: 10.1200/JCO.2011.36.4091. Epub 2011 Jul 5. J Clin Oncol. 2011. PMID: 21730267 No abstract available.
Similar articles
-
A phase 2 study of everolimus combined with trastuzumab and paclitaxel in patients with HER2-overexpressing advanced breast cancer that progressed during prior trastuzumab and taxane therapy.Breast Cancer Res Treat. 2013 Oct;141(3):437-46. doi: 10.1007/s10549-013-2689-5. Epub 2013 Oct 8. Breast Cancer Res Treat. 2013. PMID: 24101324 Free PMC article. Clinical Trial.
-
Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer.Breast Cancer Res Treat. 2011 Jan;125(2):447-55. doi: 10.1007/s10549-010-1260-x. Epub 2010 Nov 25. Breast Cancer Res Treat. 2011. PMID: 21107682 Clinical Trial.
-
Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial.Lancet Oncol. 2015 Jul;16(7):816-29. doi: 10.1016/S1470-2045(15)00051-0. Epub 2015 Jun 16. Lancet Oncol. 2015. PMID: 26092818 Clinical Trial.
-
Everolimus: a new hope for patients with breast cancer.Curr Med Res Opin. 2014 Jan;30(1):75-87. doi: 10.1185/03007995.2013.846253. Epub 2013 Oct 14. Curr Med Res Opin. 2014. PMID: 24050600 Review.
-
Evolving strategies for overcoming resistance to HER2-directed therapy: targeting the PI3K/Akt/mTOR pathway.Clin Breast Cancer. 2010 Nov;10 Suppl 3:S72-8. doi: 10.3816/CBC.2010.s.015. Clin Breast Cancer. 2010. PMID: 21115425 Review.
Cited by
-
The phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target in breast cancer.J Natl Compr Canc Netw. 2013 Jun 1;11(6):670-8. doi: 10.6004/jnccn.2013.0086. J Natl Compr Canc Netw. 2013. PMID: 23744866 Free PMC article. Review.
-
Differentiating mTOR inhibitors in renal cell carcinoma.Cancer Treat Rev. 2013 Nov;39(7):709-19. doi: 10.1016/j.ctrv.2012.12.015. Epub 2013 Feb 21. Cancer Treat Rev. 2013. PMID: 23433636 Free PMC article. Review.
-
Trastuzumab: a novel standard option for patients with HER-2-positive advanced gastric or gastro-oesophageal junction cancer.Therap Adv Gastroenterol. 2012 Sep;5(5):301-18. doi: 10.1177/1756283X12450246. Therap Adv Gastroenterol. 2012. PMID: 22973416 Free PMC article.
-
New developments in the treatment of HER2-positive breast cancer.Breast Cancer (Dove Med Press). 2012 May 1;4:53-64. doi: 10.2147/BCTT.S24976. Breast Cancer (Dove Med Press). 2012. PMID: 23869176 Free PMC article.
-
The mTOR signalling pathway in human cancer.Int J Mol Sci. 2012;13(2):1886-1918. doi: 10.3390/ijms13021886. Epub 2012 Feb 10. Int J Mol Sci. 2012. PMID: 22408430 Free PMC article. Review.
References
-
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
-
- Nahta R, Yu D, Hung MC, et al. Mechanisms of disease: Understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. - PubMed
-
- Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2–overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. - PubMed
-
- Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

