Metabotropic glutamate receptor mGluR5 is not involved in the early hemodynamic response
- PMID: 21731033
- PMCID: PMC3185891
- DOI: 10.1038/jcbfm.2011.96
Metabotropic glutamate receptor mGluR5 is not involved in the early hemodynamic response
Abstract
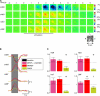
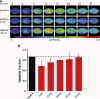
Activation of astrocytic metabotropic glutamate receptor 5 (mGluR5) is postulated to elicit calcium transients, triggering a chain of events that ultimately regulates cerebral blood flow by changing the tone of smooth muscle cells of nearby arterioles. Using concurrent in vivo optical imaging and determination of receptor occupancy with (11)C-ABP688, we report here that blocking ∼80% of mGluR5 in vivo does not affect transient hemodynamic responses on brief whisker stimulations while transiently reducing neuronal activity as measured by voltage-sensitive dye imaging. Our results show that mechanisms other than activation of mGluR5 are required to trigger the initial hemodynamic response in normal physiological conditions.
Figures


References
-
- Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12:2333–2344. - PubMed
-
- Biber K, Laurie DJ, Berthele A, Sommer B, Tolle TR, Gebicke-Harter PJ, van Calker D, Boddeke HWGM. Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J Neurochem. 1999;72:1671–1680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

