Heterogeneity in the penumbra
- PMID: 21731034
- PMCID: PMC3185890
- DOI: 10.1038/jcbfm.2011.93
Heterogeneity in the penumbra
Abstract
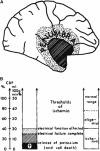
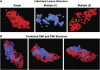
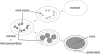
Original experimental studies in nonhuman primate models of focal ischemia showed flow-related changes in evoked potentials that suggested a circumferential zone of low regional cerebral blood flow with normal K(+) homeostasis, around a core of permanent injury in the striatum or the cortex. This became the basis for the definition of the ischemic penumbra. Imaging techniques of the time suggested a homogeneous core of injury, while positing a surrounding 'penumbral' region that could be salvaged. However, both molecular studies and observations of vascular integrity indicate a more complex and dynamic situation in the ischemic core that also changes with time. The microvascular, cellular, and molecular events in the acute setting are compatible with heterogeneity of the injury within the injury center, which at early time points can be described as multiple 'mini-cores' associated with multiple 'mini-penumbras'. These observations suggest the progression of injury from many small foci to a homogeneous defect over time after the onset of ischemia. Recent observations with updated imaging techniques and data processing support these dynamic changes within the core and the penumbra in humans following focal ischemia.
Figures




References
-
- Abumiya T, Lucero J, Heo JH, Tagaya M, Koziol JA, Copeland BR, del Zoppo GJ. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:1038–1050. - PubMed
-
- Ackerman RH, Correia JA, Alpert NM, Baron JC, Gouliamos A, Grotta JC, Brownell GL, Taveras JM. Positron imaging in ischemic stroke disease using compounds labeled with oxygen 15. Initial results of clinicophysiologic correlations. Arch Neurol. 1981;38:537–543. - PubMed
-
- Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. - PubMed
-
- Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723–725. - PubMed
-
- Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977;8:51–57. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS038710/NS/NINDS NIH HHS/United States
- R01 NS054652/NS/NINDS NIH HHS/United States
- NS39325/NS/NINDS NIH HHS/United States
- R01 NS066845/NS/NINDS NIH HHS/United States
- R01 NS053716/NS/NINDS NIH HHS/United States
- R01 NS039325/NS/NINDS NIH HHS/United States
- NS054652/NS/NINDS NIH HHS/United States
- NS 038710/NS/NINDS NIH HHS/United States
- NS044848/NS/NINDS NIH HHS/United States
- K24 NS044848/NS/NINDS NIH HHS/United States
- NS066845/NS/NINDS NIH HHS/United States
- R01 NS026945/NS/NINDS NIH HHS/United States
- R37 NS038710/NS/NINDS NIH HHS/United States
- NS 053716/NS/NINDS NIH HHS/United States
- NS 036945/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources

