Biologically Relevant Metal-Cation Binding Induces Conformational Changes in Heparin Oligosaccharides as Measured by Ion Mobility Mass Spectrometry
- PMID: 21731426
- PMCID: PMC3124288
- DOI: 10.1016/j.ijms.2011.02.003
Biologically Relevant Metal-Cation Binding Induces Conformational Changes in Heparin Oligosaccharides as Measured by Ion Mobility Mass Spectrometry
Abstract
Heparin interacts with many proteins and is involved in biological processes such as anticoagulation, angiogenesis, and antitumorigenic activities. These heparin-protein interactions can be influenced by the binding of various metal ions to these complexes. In particular, physiologically relevant metal cations influence heparin-protein conformations through electronic interactions inherent to this polyanion. In this study, we employed ion mobility mass spectrometry (IMMS) to observe conformational changes that occur in fully-sulfated heparin octasaccharides after the successive addition of metal ions. Our results indicate that binding of positive counter ions causes a decrease in collision cross section (CCS) measurements, thus promoting a more compact octasaccharide structure.
Figures
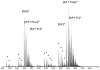

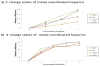
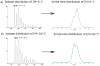


References
-
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. - PubMed
-
- Varki A. Essentials of glycobiology. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y: 2009. - PubMed
-
- Gandhi NS, Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des. 2008;72:455–482. - PubMed
-
- Powell AK, Yates EA, Fernig DG, Turnbull JE. Interactions of heparin/heparan sulfate with proteins: Appraisal of structural factors and epxerimental approaches. Glycobiology. 2004;14:17r–30r. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
