Extracellular matrix dynamics in hepatocarcinogenesis: a comparative proteomics study of PDGFC transgenic and Pten null mouse models
- PMID: 21731504
- PMCID: PMC3121762
- DOI: 10.1371/journal.pgen.1002147
Extracellular matrix dynamics in hepatocarcinogenesis: a comparative proteomics study of PDGFC transgenic and Pten null mouse models
Abstract
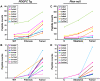
We are reporting qualitative and quantitative changes of the extracellular matrix (ECM) and associated receptor proteomes, occurring during the transition from liver fibrosis and steatohepatitis to hepatocellular carcinoma (HCC). We compared two mouse models relevant to human HCC: PDGFC transgenic (Tg) and Pten null mice, models of disease progression from fibrosis and steatohepatitis to HCC. Using mass spectrometry, we identified in the liver of both models proteins for 26 collagen-encoding genes, providing the first evidence of expression at the protein level for 16 collagens. We also identified post-transcriptional protein variants for six collagens and lysine hydroxylation modifications for 14 collagens. Tumor-associated collagen proteomes were similar in both models with increased expression of collagens type IV, VI, VII, X, XIV, XV, XVI, and XVIII. Splice variants for Col4a2, Col6a2, Col6a3 were co-upregulated while only the short form of Col18a1 increased in the tumors. We also identified tumor specific increases of nidogen 1, decorin, perlecan, and of six laminin subunits. The changes in these non-collagenous ECM proteins were similar in both models with the exception of laminin β3, detected specifically in the Pten null tumors. Pdgfa and Pdgfc mRNA expression was increased in the Pten null liver, a possible mechanism for the similarity in ECM composition observed in the tumors of both models. In contrast and besides the strong up-regulation of integrin α5 protein observed in the liver tumors of both models, the expression of the six other integrins identified was specific to each model, with integrins α2b, α3, α6, and β1 up-regulated in Pten null tumors and integrins α8 and β5 up-regulated in the PDGFC Tg tumors. In conclusion, HCC-associated ECM proteins and ECM-integrin networks, common or specific to HCC subtypes, were identified, providing a unique foundation to using ECM composition for HCC classification, diagnosis, prevention, or treatment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






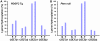

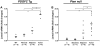





References
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- Reigstad LJ, Varhaug JE, Lillehaug JR. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J. 2005;272:5723–5741. - PubMed
-
- Fausto N, Campbell JS. Mouse models of hepatocellular carcinoma. Semin Liver Dis. 2010;30:87–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

