Simvastatin prevents dopaminergic neurodegeneration in experimental parkinsonian models: the association with anti-inflammatory responses
- PMID: 21731633
- PMCID: PMC3120752
- DOI: 10.1371/journal.pone.0020945
Simvastatin prevents dopaminergic neurodegeneration in experimental parkinsonian models: the association with anti-inflammatory responses
Abstract
Background: In addition to their original applications to lowering cholesterol, statins display multiple neuroprotective effects. N-methyl-D-aspartate (NMDA) receptors interact closely with the dopaminergic system and are strongly implicated in therapeutic paradigms of Parkinson's disease (PD). This study aims to investigate how simvastatin impacts on experimental parkinsonian models via regulating NMDA receptors.
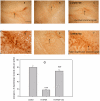
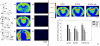
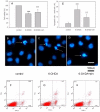
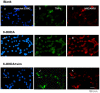
Methodology/principal findings: Regional changes in NMDA receptors in the rat brain and anxiolytic-like activity were examined after unilateral medial forebrain bundle lesion by 6-hydroxydopamine via a 3-week administration of simvastatin. NMDA receptor alterations in the post-mortem rat brain were detected by [³H]MK-801(Dizocilpine) binding autoradiography. 6-hydroxydopamine treated PC12 was applied to investigate the neuroprotection of simvastatin, the association with NMDA receptors, and the anti-inflammation. 6-hydroxydopamine induced anxiety and the downregulation of NMDA receptors in the hippocampus, CA1(Cornu Ammonis 1 Area), amygdala and caudate putamen was observed in 6-OHDA(6-hydroxydopamine) lesioned rats whereas simvastatin significantly ameliorated the anxiety-like activity and restored the expression of NMDA receptors in examined brain regions. Significant positive correlations were identified between anxiolytic-like activity and the restoration of expression of NMDA receptors in the hippocampus, amygdala and CA1 following simvastatin administration. Simvastatin exerted neuroprotection in 6-hydroxydopamine-lesioned rat brain and 6-hydroxydopamine treated PC12, partially by regulating NMDA receptors, MMP9 (matrix metalloproteinase-9), and TNF-a (tumour necrosis factor-alpha).
Conclusions/significance: Our results provide strong evidence that NMDA receptor modulation after simvastatin treatment could partially explain its anxiolytic-like activity and anti-inflammatory mechanisms in experimental parkinsonian models. These findings contribute to a better understanding of the critical roles of simvastatin in treating PD via NMDA receptors.
Conflict of interest statement
Figures



References
-
- Becker C, Jick SS, Meier CR. Use of statins and the risk of Parkinson's disease: a retrospective case-control study in the UK. Drug Saf. 2008;31(5):399–407. - PubMed
-
- Wang Q, Yan J, Chen X, Li J, Yang Y, et al. Statins: Multiple neuroprotective mechanisms in neurodegenerative diseases. Exp Neurol. 2010 In Press. - PubMed
-
- Janssen WG, Vissavajjhala P, Andrews G, Moran T, Hof PR, et al. Cellular and synaptic distribution of NR2A and NR2B in macaque monkey and rat hippocampus as visualized with subunit-specific monoclonal antibodies. Exp Neurol. 2005;191(Suppl 1):S28–44. - PubMed
-
- Nilsson A, Eriksson M, Muly EC, Akesson E, Samuelsson EB, et al. Analysis of NR3A receptor subunits in human native NMDA receptors. Brain Res. 2007;1186:102–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

