Transformation of human mesenchymal cells and skin fibroblasts into hematopoietic cells
- PMID: 21731684
- PMCID: PMC3120836
- DOI: 10.1371/journal.pone.0021250
Transformation of human mesenchymal cells and skin fibroblasts into hematopoietic cells
Abstract
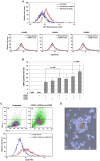
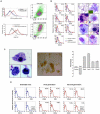
Patients with prolonged myelosuppression require frequent platelet and occasional granulocyte transfusions. Multi-donor transfusions induce alloimmunization, thereby increasing morbidity and mortality. Therefore, an autologous or HLA-matched allogeneic source of platelets and granulocytes is needed. To determine whether nonhematopoietic cells can be reprogrammed into hematopoietic cells, human mesenchymal stromal cells (MSCs) and skin fibroblasts were incubated with the demethylating agent 5-azacytidine (Aza) and the growth factors (GF) granulocyte-macrophage colony-stimulating factor and stem cell factor. This treatment transformed MSCs to round, non-adherent cells expressing T-, B-, myeloid-, or stem/progenitor-cell markers. The transformed cells engrafted as hematopoietic cells in bone marrow of immunodeficient mice. DNA methylation and mRNA array analysis suggested that Aza and GF treatment demethylated and activated HOXB genes. Indeed, transfection of MSCs or skin fibroblasts with HOXB4, HOXB5, and HOXB2 genes transformed them into hematopoietic cells. Further studies are needed to determine whether transformed MSCs or skin fibroblasts are suitable for therapy.
Conflict of interest statement
Figures
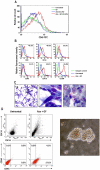
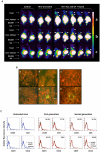
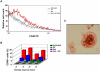
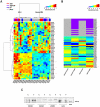
References
-
- Freireich EJ. Supportive care for patients with blood disorders. Br J Haematol. 2000;111:68–77. - PubMed
-
- Jendiroba DB, Freireich EJ. Granulocyte transfusions: from neutrophil replacement to immunereconstitution. Blood Rev. 2000;14:219–227. - PubMed
-
- Rebulla P. A mini-review on platelet refractoriness. Haematologica. 2005;90:247–253. - PubMed
-
- Sachs UJ. The pathogenesis of transfusion-related acute lung injury and how to avoid this serious adverse reaction of transfusion. Transfus Apher Sci. 2007;37:273–282. - PubMed
-
- Winters JL. Complications of donor apheresis. J Clin Apher. 2006;21:132–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

