Expression of enzymatically inactive wasp venom phospholipase A1 in Pichia pastoris
- PMID: 21731687
- PMCID: PMC3121754
- DOI: 10.1371/journal.pone.0021267
Expression of enzymatically inactive wasp venom phospholipase A1 in Pichia pastoris
Abstract
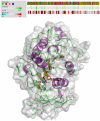
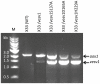
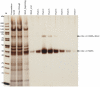
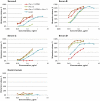
Wasp venom allergy is the most common insect venom allergy in Europe. It is manifested by large local reaction or anaphylactic shock occurring after a wasp sting. The allergy can be treated by specific immunotherapy with whole venom extracts. Wasp venom is difficult and costly to obtain and is a subject to composition variation, therefore it can be advantageous to substitute it with a cocktail of recombinant allergens. One of the major venom allergens is phospholipase A1, which so far has been expressed in Escherichia coli and in insect cells. Our aim was to produce the protein in secreted form in yeast Pichia pastoris, which can give high yields of correctly folded protein on defined minimal medium and secretes relatively few native proteins simplifying purification.Residual amounts of enzymatically active phospholipase A1 could be expressed, but the venom protein had a deleterious effect on growth of the yeast cells. To overcome the problem we introduced three different point mutations at the critical points of the active site, where serine137, aspartate165 or histidine229 were replaced by alanine (S137A, D165A and H229A). All the three mutated forms could be expressed in P. pastoris. The H229A mutant did not have any detectable phospholipase A1 activity and was secreted at the level of several mg/L in shake flask culture. The protein was purified by nickel-affinity chromatography and its identity was confirmed by MALDI-TOF mass spectrometry. The protein could bind IgE antibodies from wasp venom allergic patients and could inhibit the binding of wasp venom to IgE antibodies specific for phospholipase A1 as shown by Enzyme Allergo-Sorbent Test (EAST). Moreover, the recombinant protein was allergenic in a biological assay as demonstrated by its capability to induce histamine release of wasp venom-sensitive basophils.The recombinant phospholipase A1 presents a good candidate for wasp venom immunotherapy.
Conflict of interest statement
Figures





References
-
- Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439–447; quiz 448. doi: 10.1016/j.jaci.2005.01.005. - DOI - PubMed
-
- Charpin D, Birnbaum J, Lanteaume A, Vervloet D. Prevalence of allergy to hymenoptera stings in different samples of the general population. J Allergy Clin Immunol. 1992;90:331–334. - PubMed
-
- Mosbech H. Anaphylaxis to insect venom. Novartis Found Symp. 2004;257:177–188. discussion 188–192, 207–210, 276–285. - PubMed
-
- Crameri R, Rhyner C. Novel vaccines and adjuvants for allergen-specific immunotherapy. Curr Opin Immunol. 2006;18:761–768. doi: 10.1016/j.coi.2006.09.001. - DOI - PubMed
-
- King TP, Alagon AC, Kuan J, Sobotka AK, Lichtenstein LM. Immunochemical studies of yellowjacket venom proteins. Mol Immunol. 1983;20:297–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

