Tumor initiating cells in esophageal squamous cell carcinomas express high levels of CD44
- PMID: 21731740
- PMCID: PMC3123317
- DOI: 10.1371/journal.pone.0021419
Tumor initiating cells in esophageal squamous cell carcinomas express high levels of CD44
Abstract
Background: Esophageal Squamous Cell Carcinoma (ESCC) is a major subtype of esophageal cancer causing significant morbility and mortality in Asia. Mechanism of initiation and progression of this disease is unclear. Tumor initiating cells (TICs) are the subpopulation of cells which have the ability to self-renew, as well as, to drive initiation and progression of cancer. Increasing evidence has shown that TICs exist in a variety of tumors. However, the identification and characterization of TICs in esophageal carcinoma has remained elusive.
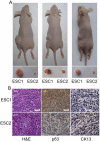
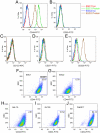
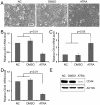
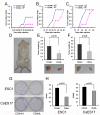
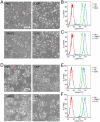
Methodology/principal findings: to identify TICs in ESCC, ESCC cell lines including two primary cells were used for screening suitable surface marker. Then colony formation assay, drug resistant assay and tumorigenicity assay in immune deficient mice were used to characterize TICs in ESCC. We found that just the CD44 expression correlated with tumorigenicity in ESCC cell lines. And then induced differentiation of ESCC cells by all-trans retinoic acid treatment led to decreased expression of CD44. The FACS isolated cell subpopulations with high CD44 expression showed increased colony formation and drug resistance in vitro, as well as significantly enhanced tumorigenicity in NOD/SICD mice, as compared to the low expressing CD44 ESCC cells.
Conclusions/significance: our study has discovered a novel TIC surface marker, CD44, which can be utilized to enrich efficiently the TICs in ESCC. These findings will be useful for further studies of these cells and exploring therapeutic approaches.
Conflict of interest statement
Figures

References
-
- Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973–1995. Int J Cancer. 2002;99:860–868. - PubMed
-
- Ke L. Mortality and incidence trends from esophagus cancer in selected geographic areas of China circa 1970–90. Int J Cancer. 2002;102:271–274. - PubMed
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

