Signal transduction in vasculogenesis and developmental angiogenesis
- PMID: 21732275
- PMCID: PMC4075038
- DOI: 10.1387/ijdb.103213sp
Signal transduction in vasculogenesis and developmental angiogenesis
Abstract
The vasculature is a highly specialized organ that functions in a number of key physiological tasks including the transport of oxygen and nutrients to tissues. Formation of the vascular system is an essential and rate-limiting step in development and occurs primarily through two main mechanisms, vasculogenesis and angiogenesis. Both vasculogenesis, the de novo formation of vessels, and angiogenesis, the growth of new vessels from pre-existing vessels by sprouting, are complex processes that are mediated by the precise coordination of multiple cell types to form and remodel the vascular system. A host of signaling molecules and their interaction with specific receptors are central to activating and modulating vessel formation. This review article summarizes the current state of research involving signaling molecules that have been demonstrated to function in the regulation of vasculogenesis and angiogenesis, as well as molecules known to play a role in vessel maturation, hypoxia-driven angiogenesis and arterial-venous specification.
Figures

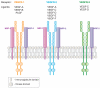
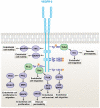
Similar articles
-
The role of angiogenic growth factors in organogenesis.Int J Dev Biol. 2011;55(4-5):365-75. doi: 10.1387/ijdb.103214ec. Int J Dev Biol. 2011. PMID: 21858761 Review.
-
Possible novel targets for therapeutic angiogenesis.Curr Opin Pharmacol. 2009 Apr;9(2):102-8. doi: 10.1016/j.coph.2008.11.006. Epub 2008 Dec 13. Curr Opin Pharmacol. 2009. PMID: 19071062 Free PMC article. Review.
-
Neuropilins in physiological and pathological angiogenesis.J Pathol. 2007 Jul;212(3):237-48. doi: 10.1002/path.2182. J Pathol. 2007. PMID: 17503412 Review.
-
Cerebral angiogenesis during development: who is conducting the orchestra?Methods Mol Biol. 2014;1135:3-20. doi: 10.1007/978-1-4939-0320-7_1. Methods Mol Biol. 2014. PMID: 24510850 Review.
-
The therapeutic potential of oxygen tension manipulation via hypoxia inducible factors and mimicking agents in guided bone regeneration. A review.Arch Oral Biol. 2011 Dec;56(12):1466-75. doi: 10.1016/j.archoralbio.2011.05.001. Epub 2011 May 28. Arch Oral Biol. 2011. PMID: 21621191 Review.
Cited by
-
Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression.Signal Transduct Target Ther. 2020 Oct 19;5(1):242. doi: 10.1038/s41392-020-00359-5. Signal Transduct Target Ther. 2020. PMID: 33077737 Free PMC article. Review.
-
Two distinct types of the inhibition of vasculogenesis by different species of charged particles.Vasc Cell. 2013 Sep 17;5(1):16. doi: 10.1186/2045-824X-5-16. Vasc Cell. 2013. PMID: 24044765 Free PMC article.
-
Repetitive myocardial ischemia promotes coronary growth in the adult mammalian heart.J Am Heart Assoc. 2013 Sep 30;2(5):e000343. doi: 10.1161/JAHA.113.000343. J Am Heart Assoc. 2013. PMID: 24080909 Free PMC article.
-
Scaffold vascularization method using an adipose-derived stem cell (ASC)-seeded scaffold prefabricated with a flow-through pedicle.Stem Cell Res Ther. 2020 Jan 23;11(1):34. doi: 10.1186/s13287-019-1535-z. Stem Cell Res Ther. 2020. PMID: 31973733 Free PMC article.
-
Interleukin-8: A potent promoter of angiogenesis in gastric cancer.Oncol Lett. 2016 Feb;11(2):1043-1050. doi: 10.3892/ol.2015.4035. Epub 2015 Dec 15. Oncol Lett. 2016. PMID: 26893688 Free PMC article.
References
-
- Aase K, von Euler G, Li X, Ponten A, Thoren P, Cao R, Cao Y, Olofsson B, Gebre-Medhin S, Pekny M, et al. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104:358–64. - PubMed
-
- Ahmed Z, Bicknell R. Angiogenic signalling pathways. Methods Mol Biol. 2009;467:3–24. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Azuma H. Genetic and molecular pathogenesis of hereditary hemorrhagic telangiectasia. J Med Invest. 2000;47:81–90. - PubMed
-
- Baird A, Durkin T. Inhibition of endothelial cell proliferation by type beta-transforming growth factor: interations with acidic and basic fibroblast growth factors. Biochem. Biophys. Res. Commun. 1986;138:476–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

