Requirements for ion and solute transport, and pH regulation during enamel maturation
- PMID: 21732355
- PMCID: PMC3373187
- DOI: 10.1002/jcp.22911
Requirements for ion and solute transport, and pH regulation during enamel maturation
Abstract
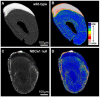
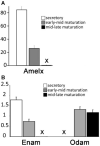
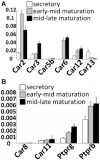
Transcellular bicarbonate transport is suspected to be an important pathway used by ameloblasts to regulate extracellular pH and support crystal growth during enamel maturation. Proteins that play a role in amelogenesis include members of the ABC transporters (SLC gene family and CFTR). A number of carbonic anhydrases (CAs) have also been identified. The defined functions of these genes are likely interlinked during enamel mineralization. The purpose of this study is to quantify relative mRNA levels of individual SLC, Cftr, and CAs in enamel cells obtained from secretory and maturation stages on rat incisors. We also present novel data on the enamel phenotypes for two animal models, a mutant porcine (CFTR-ΔF508) and the NBCe1-null mouse. Our data show that two SLCs (AE2 and NBCe1), Cftr, and Car2, Car3, Car6, and Car12 are all significantly up-regulated at the onset of the maturation stage of amelogenesis when compared to the secretory stage. The remaining SLCs and CA gene transcripts showed negligible expression or no significant change in expression from secretory to maturation stages. The enamel of CFTR-ΔF508 adult pigs was hypomineralized and showed abnormal crystal growth. NBCe1-null mice enamel was structurally defective and had a marked decrease in mineral content relative to wild-type. These data demonstrate the importance of many non-matrix proteins to amelogenesis and that the expression levels of multiple genes regulating extracellular pH are modulated during enamel maturation in response to an increased need for pH buffering during hydroxyapatite crystal growth.
Copyright © 2011 Wiley Periodicals, Inc.
Figures



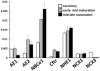

References
-
- Aramaki S, Yoshida I, Yoshino M, Kondo M, Sato Y, Noda K, Jo R, Okue A, Sai N, Yamashita F. Carbonic anhydrase II deficiency in three unrelated Japanese patients. J Inherit Metab Dis. 1993;16:982–990. - PubMed
-
- Arquitt CK, Boyd C, Wright JT. Cystic fibrosis transmembrane regulator gene (CFTR) is associated with abnormal enamel formation. J Dent Res. 2002;81:492–496. - PubMed
-
- Atar M, Korperich EJ. Systemic disorders and their influence on the development of dental hard tissues: A literature review. J Dent. 2010;38:296–306. - PubMed
-
- Awad M, Al-Ashwal AA, Sakati N, Al-Abbad AA, Bin-Abbas BS. Long-term follow up of carbonic anhydrase II deficiency syndrome. Saudi Med J. 2002;23:25–29. - PubMed
-
- Azevedo TD, Feijo GC, Bezerra AC. Presence of developmental defects of enamel in cystic fibrosis patients. J Dent Child (Chic) 2006;73:159–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE019629/DE/NIDCR NIH HHS/United States
- HL051670/HL/NHLBI NIH HHS/United States
- P01 HL091842/HL/NHLBI NIH HHS/United States
- P01 HL051670/HL/NHLBI NIH HHS/United States
- CAPMC/ CIHR/Canada
- R01 DK077162/DK/NIDDK NIH HHS/United States
- HL091842/HL/NHLBI NIH HHS/United States
- K08 DE021413/DE/NIDCR NIH HHS/United States
- R01 DK058563/DK/NIDDK NIH HHS/United States
- DK077162/DK/NIDDK NIH HHS/United States
- DE013404/DE/NIDCR NIH HHS/United States
- R01 DE013404/DE/NIDCR NIH HHS/United States
- DE019629/DE/NIDCR NIH HHS/United States
- DK058563/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

