New regimens to prevent tuberculosis in adults with HIV infection
- PMID: 21732833
- PMCID: PMC3407678
- DOI: 10.1056/NEJMoa1005136
New regimens to prevent tuberculosis in adults with HIV infection
Abstract
Background: Treatment of latent tuberculosis in patients infected with the human immunodeficiency virus (HIV) is efficacious, but few patients around the world receive such treatment. We evaluated three new regimens for latent tuberculosis that may be more potent and durable than standard isoniazid treatment.
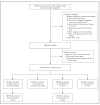
Methods: We randomly assigned South African adults with HIV infection and a positive tuberculin skin test who were not taking antiretroviral therapy to receive rifapentine (900 mg) plus isoniazid (900 mg) weekly for 12 weeks, rifampin (600 mg) plus isoniazid (900 mg) twice weekly for 12 weeks, isoniazid (300 mg) daily for up to 6 years (continuous isoniazid), or isoniazid (300 mg) daily for 6 months (control group). The primary end point was tuberculosis-free survival.
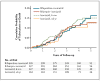
Results: The 1148 patients had a median age of 30 years and a median CD4 cell count of 484 per cubic millimeter. Incidence rates of active tuberculosis or death were 3.1 per 100 person-years in the rifapentine-isoniazid group, 2.9 per 100 person-years in the rifampin-isoniazid group, and 2.7 per 100 person-years in the continuous-isoniazid group, as compared with 3.6 per 100 person-years in the control group (P>0.05 for all comparisons). Serious adverse reactions were more common in the continuous-isoniazid group (18.4 per 100 person-years) than in the other treatment groups (8.7 to 15.4 per 100 person-years). Two of 58 isolates of Mycobacterium tuberculosis (3.4%) were found to have multidrug resistance.
Conclusions: On the basis of the expected rates of tuberculosis in this population of HIV-infected adults, all secondary prophylactic regimens were effective. Neither a 3-month course of intermittent rifapentine or rifampin with isoniazid nor continuous isoniazid was superior to 6 months of isoniazid. (Funded by the National Institute of Allergy and Infectious Diseases and others; ClinicalTrials.gov number, NCT00057122.).
Conflict of interest statement
Dr. Martinson reports receiving lecture fees from Alere. No other potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
What is thwarting tuberculosis prevention in high-burden settings?N Engl J Med. 2011 Jul 7;365(1):79-81. doi: 10.1056/NEJMe1105555. N Engl J Med. 2011. PMID: 21732840 No abstract available.
-
Nuevas pautas para la prevención de tuberculosis en adultos con infección VIH.Rev Clin Esp. 2012 Feb;212(2):104. doi: 10.1016/j.rce.2011.09.006. Rev Clin Esp. 2012. PMID: 22413133 Spanish. No abstract available.
References
-
- Global tuberculosis control: epidemiology, strategy, financing: WHO report 2009. Geneva: World Health Organization; 2009.
-
- Whalen CC, Johnson JL, Okwera A, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. N Engl J Med. 1997;337:801–8. - PubMed
-
- Pape JW, Jean SS, Ho JL, Hafner A, Johnson WD., Jr Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–72. - PubMed
-
- Mwinga A, Hosp M, Godfrey-Faussett P, et al. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. AIDS. 1998;12:2447–57. - PubMed
-
- Bucher HC, Griffith LE, Guyatt GH, et al. Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. AIDS. 1999;13:501–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
