Irinotecan induces steroid and xenobiotic receptor (SXR) signaling to detoxification pathway in colon cancer cells
- PMID: 21733184
- PMCID: PMC3144021
- DOI: 10.1186/1476-4598-10-80
Irinotecan induces steroid and xenobiotic receptor (SXR) signaling to detoxification pathway in colon cancer cells
Abstract
Background: Resistance to chemotherapy remains one of the principle obstacles to the treatment of colon cancer. In order to identify the molecular mechanism of this resistance, we investigated the role of the steroid and xenobiotic receptor (SXR) in the induction of drug resistance. Indeed, this nuclear receptor plays an important role in response to xenobiotics through the upregulation of detoxification genes. Following drug treatments, SXR is activated and interacts with the retinoid X receptor (RXR) to induce expression of some genes involved in drug metabolism such as phase I enzyme (like CYP), phase II enzymes (like UGT) and transporters (e.g. MDR1).
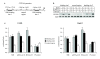
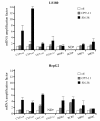
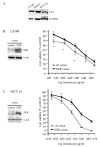
Results: In this study, we have shown that endogenous SXR is activated in response to SN-38, the active metabolite of the anticancer drug irinotecan, in human colon cancer cell lines. We have found that endogenous SXR translocates into the nucleus and associates with RXR upon SN-38 treatment. Using ChIP, we have demonstrated that endogenous SXR, following its activation, binds to the native promoter of the CYP3A4 gene to induce its expression. RNA interference experiments confirmed SXR involvement in CYP3A4 overexpression and permitted us to identify CYP3A5 and MRP2 transporter as SXR target genes. As a consequence, cells overexpressing SXR were found to be less sensitive to irinotecan treatment.
Conclusions: Altogether, these results suggest that the SXR pathway is involved in colon cancer irinotecan resistance in colon cancer cell line via the upregulation of select detoxification genes.
Figures


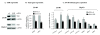
Similar articles
-
Tamoxifen activates CYP3A4 and MDR1 genes through steroid and xenobiotic receptor in breast cancer cells.Endocrine. 2006 Dec;30(3):261-8. doi: 10.1007/s12020-006-0003-6. Endocrine. 2006. PMID: 17526937
-
The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism.Nucl Recept Signal. 2009;7:e001. doi: 10.1621/nrs.07001. Epub 2009 Jan 16. Nucl Recept Signal. 2009. PMID: 19240808 Free PMC article. Review.
-
Putative role of the orphan nuclear receptor SXR (steroid and xenobiotic receptor) in the mechanism of CYP3A4 inhibition by xenobiotics.J Biol Chem. 2002 Sep 6;277(36):32453-8. doi: 10.1074/jbc.M111245200. Epub 2002 Jun 18. J Biol Chem. 2002. PMID: 12072427
-
Rexinoids modulate steroid and xenobiotic receptor activity by increasing its protein turnover in a calpain-dependent manner.J Biol Chem. 2008 Aug 8;283(32):21945-52. doi: 10.1074/jbc.M710358200. Epub 2008 Jun 9. J Biol Chem. 2008. PMID: 18544536
-
[The role of nuclear receptors in cytochrome P-450 induction by xenochemicals].Rocz Panstw Zakl Hig. 2002;53(4):321-32. Rocz Panstw Zakl Hig. 2002. PMID: 12664659 Review. Polish.
Cited by
-
Germline Polymorphisms in the Nuclear Receptors PXR and VDR as Novel Prognostic Markers in Metastatic Colorectal Cancer Patients Treated With FOLFIRI.Front Oncol. 2019 Nov 26;9:1312. doi: 10.3389/fonc.2019.01312. eCollection 2019. Front Oncol. 2019. PMID: 31850208 Free PMC article.
-
Cellular irinotecan resistance in colorectal cancer and overcoming irinotecan refractoriness through various combination trials including DNA methyltransferase inhibitors: a review.Cancer Drug Resist. 2021 Nov 2;4(4):946-964. doi: 10.20517/cdr.2021.82. eCollection 2021. Cancer Drug Resist. 2021. PMID: 35582377 Free PMC article. Review.
-
Pregnane X receptor is associated with unfavorable survival and induces chemotherapeutic resistance by transcriptional activating multidrug resistance-related protein 3 in colorectal cancer.Mol Cancer. 2017 Mar 29;16(1):71. doi: 10.1186/s12943-017-0641-8. Mol Cancer. 2017. PMID: 28356150 Free PMC article.
-
The Multifarious Link between Cytochrome P450s and Cancer.Oxid Med Cell Longev. 2020 Jan 3;2020:3028387. doi: 10.1155/2020/3028387. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 31998435 Free PMC article. Review.
-
Expression of the PXR gene in various types of cancer and drug resistance.Oncol Lett. 2013 Apr;5(4):1093-1100. doi: 10.3892/ol.2013.1149. Epub 2013 Jan 22. Oncol Lett. 2013. PMID: 23599746 Free PMC article.
References
-
- Plant N. Biochim Biophys Acta. Vol. 1770. Netherlands; 2007. The human cytochrome P450 sub-family: transcriptional regulation, inter-individual variation and interaction networks; pp. 478–488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

