Functional improvement and correlations with symptomatic improvement in adults with attention deficit hyperactivity disorder receiving long-acting methylphenidate
- PMID: 21733214
- PMCID: PMC3226157
- DOI: 10.1017/S0033291711000845
Functional improvement and correlations with symptomatic improvement in adults with attention deficit hyperactivity disorder receiving long-acting methylphenidate
Abstract
Background: Data on the relationship between core symptoms and daily functioning in adults with attention deficit hyperactivity disorder (ADHD) are limited. Daily functioning was assessed as part of an open-label extension, and associations with symptom scores were evaluated.
Method: After a 5-week double-blind study with adults with ADHD receiving osmotic-controlled release oral delivery system (OROS) methylphenidate (MPH) 18, 36 or 72 mg/day, or placebo, participants were eligible for a 7-week open-label extension in which all patients received OROS MPH. Data for the Conners' Adult ADHD Rating Scale - Observer: Screening Version (CAARS-O:SV) (primary endpoint) have been presented previously. Secondary endpoints included the observer self-reported short version of the CAARS (CAARS-S:S) and the Clinical Global Impressions - Severity Scale (CGI-S). Daily functioning and quality of life were assessed using the Sheehan Disability Scale (SDS) and the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) respectively. In post-hoc analyses, changes in CAARS-O:SV were evaluated in subgroups. Relationships between symptom and functional outcomes were evaluated in a multivariate regression analysis.
Results: A total of 370 patients entered the open-label extension. Significant improvements from baseline in CAARS-O:SV were similar regardless of sex, ADHD subtype, prior treatment or psychiatric co-morbidity. Significant improvements from double-blind baseline were also seen for the CAARS-S:S, CGI-S, SDS and Q-LES-Q. Improvements in the CAARS-O:SV Hyperactivity/Impulsivity subscale were associated with improvements in SDS total and subscale scores, and in the Q-LES-Q score at open-label endpoint. Improvements in CAARS-O:SV Inattention subscale and CGI-S scores were not significantly associated with functional changes.
Conclusions: Improvements in ADHD symptoms relating to hyperactivity and impulsivity in adults receiving OROS MPH are associated with improvements in daily functioning and quality of life.
Figures
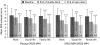
References
-
- Adler LA Spencer TJ Levine LR Ramsey JL Tamura R Kelsey D Ball SG Allen AJ Biederman J 2008aFunctional outcomes in the treatment of adults with ADHD Journal of Attention Disorders 11720–727. - PubMed
-
- Adler LA Spencer TJ Williams DW Moore RJ Michelson D 2008bLong-term, open-label safety and efficacy of atomoxetine in adults with ADHD: final report of a 4-year study Journal of Attention Disorders 12248–253. - PubMed
-
- Adler LA, Sutton VK, Moore RJ, Dietrich AP, Reimherr FW, Sangal RB, Saylor KE, Secnik K, Kelsey DK, Allen AJ. Quality of life assessment in adult patients with attention-deficit/hyperactivity disorder treated with atomoxetine. Journal of Clinical Psychopharmacology. 2006;26:648–652. - PubMed
-
- Adler LA, Zimmerman B, Starr HL, Silber S, Palumbo J, Orman C, Spencer T. Efficacy and safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, double-blind, parallel group, dose-escalation study. Journal of Clinical Psychopharmacology. 2009;29:239–247. - PubMed
-
- Arbuckle R, Frye MA, Brecher M, Paulsson B, Rajagopalan K, Palmer S, Degl'Innocenti A. The psychometric validation of the Sheehan Disability Scale (SDS) in patients with bipolar disorder. Psychiatry Research. 2009;165:163–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

