Distinct periods of developmental sensitivity to the effects of 3,4-(±)-methylenedioxymethamphetamine (MDMA) on behaviour and monoamines in rats
- PMID: 21733225
- PMCID: PMC4599583
- DOI: 10.1017/S1461145711000952
Distinct periods of developmental sensitivity to the effects of 3,4-(±)-methylenedioxymethamphetamine (MDMA) on behaviour and monoamines in rats
Abstract
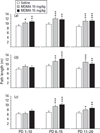
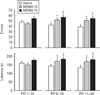
Previous findings showed allocentric and egocentric learning deficits in rats after MDMA treatment from postnatal days (PD) 11-20 but not after treatment from PD 1-10. Shorter treatment periods (PD 1-5, 6-10, 11-15, or 16-20) resulted in allocentric learning deficits averaged across intervals but not for any interval individually and no egocentric learning deficits individually or collectively. Whether this difference was attributable to treatment length or age at the start of treatment was unclear. In the present experiment rat litters were treated on PD 1-10, 6-15, or 11-20 with 0, 10, or 15 mg/kg MDMA q.i.d. at 2-h intervals. Two male/female pairs/litter received each treatment. One pair/litter received acoustic startle with prepulse inhibition, straight channel swimming, Cincinnati water maze (CWM), and conditioned fear in a latent inhibition paradigm. The other pair/litter received locomotor activity, straight channel swimming, Morris water maze (MWM), and locomotor activity retest with MK-801 challenge. MDMA impaired CWM learning following PD 6-15 or 11-20 exposure. In MWM acquisition, all MDMA-treated groups showed impairment. During reversal and shift, the PD 6-15 and PD 11-20 MDMA-treated groups were significantly impaired. Reductions in locomotor activity were most evident after PD 6-15 treatment while increases in acoustic startle were most evident after PD 1-10 treatment. After MK-801 challenge, MDMA-treated offspring showed less locomotion compared to controls. Region-specific changes in brain monoamines were also observed but were not significantly correlated with behavioural changes. The results show that PD 11-20 exposure to MDMA caused the largest long-term cognitive deficits followed by PD 6-15 exposure with PD 1-10 exposure least affected. Other effects, such as those upon MK-801-stimulated locomotion showed greatest effects after PD 1-10 MDMA exposure. Hence, each effect has a different window of developmental susceptibility.
Figures





Similar articles
-
Learning and memory after neonatal exposure to 3,4-methylenedioxymethamphetamine (ecstasy) in rats: interaction with exposure in adulthood.Synapse. 2005 Sep 1;57(3):148-59. doi: 10.1002/syn.20166. Synapse. 2005. PMID: 15945064 Free PMC article.
-
(+/-)3,4-Methylenedioxymethamphetamine (MDMA) dose-dependently impairs spatial learning in the morris water maze after exposure of rats to different five-day intervals from birth to postnatal day twenty.Dev Neurosci. 2009;31(1-2):107-20. doi: 10.1159/000207499. Epub 2009 Apr 17. Dev Neurosci. 2009. PMID: 19372692 Free PMC article.
-
Developmental effects of +/-3,4-methylenedioxymethamphetamine on spatial versus path integration learning: effects of dose distribution.Synapse. 2007 Jul;61(7):488-99. doi: 10.1002/syn.20379. Synapse. 2007. PMID: 17415794 Free PMC article.
-
3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings.Psychopharmacology (Berl). 2007 Jan;189(4):407-24. doi: 10.1007/s00213-006-0322-6. Epub 2006 Mar 16. Psychopharmacology (Berl). 2007. PMID: 16541247 Free PMC article. Review.
-
Developmental effects of 3,4-methylenedioxymethamphetamine: a review.Behav Pharmacol. 2008 Mar;19(2):91-111. doi: 10.1097/FBP.0b013e3282f62c76. Behav Pharmacol. 2008. PMID: 18332674 Free PMC article. Review.
Cited by
-
Paradoxical effects of low dose MDMA on latent inhibition in the rat.Neuropharmacology. 2013 Apr;67:331-6. doi: 10.1016/j.neuropharm.2012.11.012. Epub 2012 Nov 29. Neuropharmacology. 2013. PMID: 23201353 Free PMC article.
-
MDMA enhances hippocampal-dependent learning and memory under restrictive conditions, and modifies hippocampal spine density.Psychopharmacology (Berl). 2014 Mar;231(5):863-74. doi: 10.1007/s00213-013-3304-5. Epub 2013 Oct 26. Psychopharmacology (Berl). 2014. PMID: 24158501
-
Cincinnati water maze: A review of the development, methods, and evidence as a test of egocentric learning and memory.Neurotoxicol Teratol. 2016 Sep-Oct;57:1-19. doi: 10.1016/j.ntt.2016.08.002. Epub 2016 Aug 18. Neurotoxicol Teratol. 2016. PMID: 27545092 Free PMC article. Review.
References
-
- Bronson ME, Jiang W, Clark CR, DeRuiter J. Effects of designer drugs on the chicken embryo and 1-day-old chicken. Brain Research Bulletin. 1994;34:143–150. - PubMed
-
- Cain DP. Prior non-spatial pretraining eliminates sensorimotor disturbances and impairments in water maze learning caused by diazepam. Psychopharmacology (Berlin) 1997;130:313–319. - PubMed
-
- Clancy B, Kersh B, Hyde J, Darlington RB, et al. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007b;5:79–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

