Glucocorticoid elevation of dexamethasone-induced gene 2 (Dig2/RTP801/REDD1) protein mediates autophagy in lymphocytes
- PMID: 21733849
- PMCID: PMC3191057
- DOI: 10.1074/jbc.M111.245423
Glucocorticoid elevation of dexamethasone-induced gene 2 (Dig2/RTP801/REDD1) protein mediates autophagy in lymphocytes
Erratum in
- J Biol Chem. 2011 Nov 11;286(45):39673
Abstract
Glucocorticoid hormones, including dexamethasone, induce apoptosis in lymphocytes and consequently are used clinically as chemotherapeutic agents in many hematologic malignancies. Dexamethasone also induces autophagy in lymphocytes, although the mechanism is not fully elucidated. Through gene expression analysis, we found that dexamethasone induces the expression of a gene encoding a stress response protein variously referred to as Dig2, RTP801, or REDD1. This protein is reported to inhibit mammalian target of rapamycin (mTOR) signaling. Because autophagy is one outcome of mTOR inhibition, we investigated the hypothesis that Dig2/RTP801/REDD1 elevation contributes to autophagy induction in dexamethasone-treated lymphocytes. In support of this hypothesis, RNAi-mediated suppression of Dig2/RTP801/REDD1 reduces mTOR inhibition and autophagy in glucocorticoid-treated lymphocytes. We observed similar results in Dig2/Rtp801/Redd1 knock-out murine thymocytes treated with dexamethasone. Dig2/RTP801/REDD1 knockdown also leads to increased levels of dexamethasone-induced cell death, suggesting that Dig2/RTP801/REDD1-mediated autophagy promotes cell survival. Collectively, these findings demonstrate for the first time that elevation of Dig2/RTP801/REDD1 contributes to the induction of autophagy.
Figures
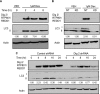
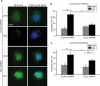
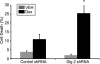
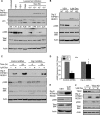
References
-
- Dougherty T. F., White A. (1943) Science 98, 367–369 - PubMed
-
- Hench P. S., Kendall E. C. (1949) Mayo Clin. Proc. 24, 181–197 - PubMed
-
- Pearson O. H., Eliel L. P. (1949) Cancer 2, 943–945 - PubMed
-
- Rhen T., Cidlowski J. A. (2005) N. Engl. J. Med. 353, 1711–1723 - PubMed
-
- Bowen D. A., Call T. G., Jenkins G. D., Zent C. S., Schwager S. M., Van Dyke D. L., Jelinek D. F., Kay N. E., Shanafelt T. D. (2007) Leuk. Lymphoma 48, 2412–2417 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

