K-Cl cotransporter gene expression during human and murine erythroid differentiation
- PMID: 21733850
- PMCID: PMC3162409
- DOI: 10.1074/jbc.M110.206516
K-Cl cotransporter gene expression during human and murine erythroid differentiation
Abstract
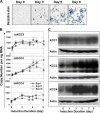
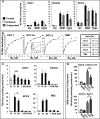
The K-Cl cotransporter (KCC) regulates red blood cell (RBC) volume, especially in reticulocytes. Western blot analysis of RBC membranes revealed KCC1, KCC3, and KCC4 proteins in mouse and human cells, with higher levels in reticulocytes. KCC content was higher in sickle versus normal RBC, but the correlation with reticulocyte count was poor, with inter-individual variability in KCC isoform ratios. Messenger RNA for each isoform was measured by real time RT-quantitative PCR. In human reticulocytes, KCC3a mRNA levels were consistently the highest, 1-7-fold higher than KCC4, the second most abundant species. Message levels for KCC1 and KCC3b were low. The ratios of KCC RNA levels varied among individuals but were similar in sickle and normal RBC. During in vivo maturation of human erythroblasts, KCC3a RNA was expressed consistently, whereas KCC1 and KCC3b levels declined, and KCC4 message first increased and then decreased. In mouse erythroblasts, a similar pattern for KCC3 and KCC1 expression during in vivo differentiation was observed, with low KCC4 RNA throughout despite the presence of KCC4 protein in mature RBC. During differentiation of mouse erythroleukemia cells, protein levels of KCCs paralleled increasing mRNA levels. Functional properties of KCCs expressed in HEK293 cells were similar to each other and to those in human RBC. However, the anion dependence of KCC in RBC resembled most closely that of KCC3. The results suggest that KCC3 is the dominant isoform in erythrocytes, with variable expression of KCC1 and KCC4 among individuals that could result in modulation of KCC activity.
Figures




Similar articles
-
Angiogenic growth factors augment K-Cl cotransporter expression in erythroid cells via hypoxia-inducible factor-1α.Am J Hematol. 2014 Mar;89(3):273-81. doi: 10.1002/ajh.23631. Am J Hematol. 2014. PMID: 24227191 Free PMC article.
-
Volume regulation and KCl cotransport in reticulocyte populations of sickle and normal red blood cells.Blood Cells Mol Dis. 2011 Aug 15;47(2):95-9. doi: 10.1016/j.bcmd.2011.04.007. Epub 2011 May 14. Blood Cells Mol Dis. 2011. PMID: 21576026 Free PMC article.
-
Multiple isoforms of the KC1 cotransporter are expressed in sickle and normal erythroid cells.Exp Hematol. 2005 Jun;33(6):624-31. doi: 10.1016/j.exphem.2005.02.006. Exp Hematol. 2005. PMID: 15911086
-
K+-Cl- cotransporter 1 (KCC1): a housekeeping membrane protein that plays key supplemental roles in hematopoietic and cancer cells.J Hematol Oncol. 2019 Jul 11;12(1):74. doi: 10.1186/s13045-019-0766-x. J Hematol Oncol. 2019. PMID: 31296230 Free PMC article. Review.
-
Regulation of K-Cl cotransport: from function to genes.J Membr Biol. 2004 Oct 1;201(3):109-37. doi: 10.1007/s00232-004-0695-6. J Membr Biol. 2004. PMID: 15711773 Review.
Cited by
-
Role of the cation-chloride-cotransporters in the circadian system.Asian J Pharm Sci. 2021 Sep;16(5):589-597. doi: 10.1016/j.ajps.2020.10.003. Epub 2020 Dec 7. Asian J Pharm Sci. 2021. PMID: 34849164 Free PMC article. Review.
-
The effect of the antisickling compound GBT1118 on the permeability of red blood cells from patients with sickle cell anemia.Physiol Rep. 2019 Mar;7(6):e14027. doi: 10.14814/phy2.14027. Physiol Rep. 2019. PMID: 30916477 Free PMC article.
-
The role of WNK in modulation of KCl cotransport activity in red cells from normal individuals and patients with sickle cell anaemia.Pflugers Arch. 2019 Dec;471(11-12):1539-1549. doi: 10.1007/s00424-019-02327-7. Epub 2019 Nov 15. Pflugers Arch. 2019. PMID: 31729557 Free PMC article.
-
Angiogenic growth factors augment K-Cl cotransporter expression in erythroid cells via hypoxia-inducible factor-1α.Am J Hematol. 2014 Mar;89(3):273-81. doi: 10.1002/ajh.23631. Am J Hematol. 2014. PMID: 24227191 Free PMC article.
-
Disorders of erythrocyte hydration.Blood. 2017 Dec 21;130(25):2699-2708. doi: 10.1182/blood-2017-04-590810. Epub 2017 Oct 19. Blood. 2017. PMID: 29051181 Free PMC article. Review.
References
-
- Gamba G. (2005) Physiol. Rev. 85, 423–493 - PubMed
-
- Gillen C. M., Brill S., Payne J. A., Forbush B., 3rd (1996) J. Biol. Chem. 271, 16237–16244 - PubMed
-
- Payne J. A., Stevenson T. J., Donaldson L. F. (1996) J. Biol. Chem. 271, 16245–16252 - PubMed
-
- Hiki K., D'Andrea R. J., Furze J., Crawford J., Woollatt E., Sutherland G. R., Vadas M. A., Gamble J. R. (1999) J. Biol. Chem. 274, 10661–10667 - PubMed
-
- Mount D. B., Mercado A., Song L., Xu J., George A. L., Jr., Delpire E., Gamba G. (1999) J. Biol. Chem. 274, 16355–16362 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

