Capture of circulatory endothelial progenitor cells and accelerated re-endothelialization of a bio-engineered stent in human ex vivo shunt and rabbit denudation model
- PMID: 21733913
- PMCID: PMC3249218
- DOI: 10.1093/eurheartj/ehr196
Capture of circulatory endothelial progenitor cells and accelerated re-endothelialization of a bio-engineered stent in human ex vivo shunt and rabbit denudation model
Abstract
Aims: The Genous™ Bio-engineered R™ stent (GS) aims to promote vascular healing by capture of circulatory endothelial progenitor cells (EPCs) to the surface of the stent struts, resulting in accelerated re-endothelialization. Here, we assessed the function of the GS in comparison to bare-metal stent (BMS), when exposed to the human and animal circulation.
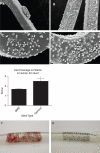
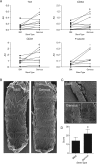
Methods and results: First, 15 patients undergoing coronary angiography received an extracorporeal femoral arteriovenous (AV) shunt containing BMS and GS. Macroscopical mural thrombi were observed in BMS, whereas GS remained visibly clean. Confocal and scanning electron microscopic (SEM) analysis of GS showed an increase in strut coverage. Quantitative polymerase chain reaction (qPCR) analysis of captured cells on the GS demonstrated increased expression of endothelial markers KDR/VEGFR2 and E-selectin, and a decrease in pro-thrombogenic markers tissue factor pathway inhibitor and plasminogen activator inhibitor-1 compared with BMS. Secondly, a similar primate AV shunt model was used to validate these findings and occlusion of BMS was observed, while GS remained patent, as demonstrated by live imaging of indium-labelled platelets. Thirdly, in an in vitro cell-capture assay, GS struts showed increased coverage by EPCs, whereas monocyte coverage remained similar to BMS. Finally, the assessment of re-endothelialization was studied in a rabbit denudation model. Twenty animals received BMS and GS in the aorta and iliac arteries for 7 days. Scanning electron microscopic analysis showed a trend towards increased strut coverage, confirmed by qPCR analysis revealing increased levels of endothelial markers (Tie2, CD34, PCD31, and P-selectin) in GS.
Conclusion: In this proof-of-concept study, we have demonstrated that the bio-engineered EPC-capture stent, Genous™ R™ stent, is effective in EPC capture, resulting in accelerated re-endothelialization and reduced thrombogenicity.
Figures


References
-
- Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. - PubMed
-
- Asahara T, Murohara T, Sullivan A. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Gulati R, Simari RD. Cell therapy for acute myocardial infarction. Med Clin North Am. 2007;91:769–785. xiii. - PubMed
-
- Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–1189. - PubMed
-
- Kipshidze N, Dangas G, Tsapenko M. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 2004;44:733–739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

