High-fat diet-induced adipocyte cell death occurs through a cyclophilin D intrinsic signaling pathway independent of adipose tissue inflammation
- PMID: 21734017
- PMCID: PMC3142076
- DOI: 10.2337/db10-1411
High-fat diet-induced adipocyte cell death occurs through a cyclophilin D intrinsic signaling pathway independent of adipose tissue inflammation
Abstract
Objective: Previous studies have demonstrated that mice fed a high-fat diet (HFD) develop insulin resistance with proinflammatory macrophage infiltration into white adipose tissue. Concomitantly, adipocytes undergo programmed cell death with the loss of the adipocyte-specific lipid droplet protein perilipin, and the dead/dying adipocytes are surrounded by macrophages that are organized into crown-like structures. This study investigated whether adipocyte cell death provides the driving signal for macrophage inflammation or if inflammation induces adipocyte cell death.
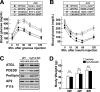
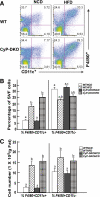
Research design and methods: Two knockout mouse models were used: granulocyte/monocyte-colony stimulating factor (GM-CSF)-null mice that are protected against HFD-induced adipose tissue inflammation and cyclophilin D (CyP-D)-null mice that are protected against adipocyte cell death. Mice were fed for 4-14 weeks with a 60% HFD, and different markers of cell death and inflammation were analyzed.
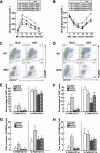
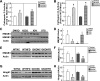
Results: HFD induced a normal extent of adipocyte cell death in GM-CSF-null mice, despite a marked reduction in adipose tissue inflammation. Similarly, depletion of macrophages by clodronate treatment prevented HFD-induced adipose tissue inflammation without any affect on adipocyte cell death. However, CyP-D deficiency strongly protected adipocytes from HFD-induced cell death, without affecting adipose tissue inflammation.
Conclusions: These data demonstrate that HFD-induced adipocyte cell death is an intrinsic cellular response that is CyP-D dependent but is independent of macrophage infiltration/activation.
Figures



References
-
- Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res 2008;49:1894–1903 - PubMed
-
- Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009;15:914–920 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

