Gastric bypass reduces fat intake and preference
- PMID: 21734019
- PMCID: PMC3197335
- DOI: 10.1152/ajpregu.00139.2011
Gastric bypass reduces fat intake and preference
Abstract
Roux-en-Y gastric bypass is the most effective therapy for morbid obesity. This study investigated how gastric bypass affects intake of and preference for high-fat food in an experimental (rat) study and within a trial setting (human). Proportion of dietary fat in gastric bypass patients was significantly lower 6 yr after surgery compared with patients after vertical-banded gastroplasty (P = 0.046). Gastric bypass reduced total fat and caloric intake (P < 0.001) and increased standard low-fat chow consumption compared with sham controls (P < 0.001) in rats. Compared with sham-operated rats, gastric bypass rats displayed much lower preferences for Intralipid concentrations > 0.5% in an ascending concentration series (0.005%, 0.01%, 0.05%, 0.1%, 0.5%, 1%, 5%) of two-bottle preference tests (P = 0.005). This effect was demonstrated 10 and 200 days after surgery. However, there was no difference in appetitive or consummatory behavior in the brief access test between the two groups (P = 0.71) using similar Intralipid concentrations (0.005% through 5%). Levels of glucagon-like peptide-1 (GLP-1) were increased after gastric bypass as expected. An oral gavage of 1 ml corn oil after saccharin ingestion in gastric bypass rats induced a conditioned taste aversion. These findings suggest that changes in fat preference may contribute to long-term maintained weight loss after gastric bypass. Postingestive effects of high-fat nutrients resulting in conditioned taste aversion may partially explain this observation; the role of GLP-1 in mediating postprandial responses after gastric bypass requires further investigation.
Figures
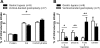


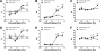
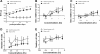
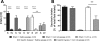
References
-
- Ahmed AR, Rickards G, Coniglio D, Xia Y, Johnson J, Boss T, O'Malley W. Laparoscopic Roux-en-Y gastric bypass and its early effect on blood pressure. Obes Surg 19: 845–849, 2009 - PubMed
-
- Allison DB, Saunders SE. Obesity in North America. An overview. Med Clin North Am 84: 305–332, 2000 - PubMed
-
- Baumgartner I, Pacheco-Lopez G, Ruttimann EB, Arnold M, Asarian L, Langhans W, Geary N, Hillebrand JJ. Hepatic-portal vein infusions of glucagon-like peptide-1 reduce meal size and increase c-Fos expression in the nucleus tractus solitarii, area postrema and central nucleus of the amygdala in rats. J Neuroendocrinol 22: 557–563, 2010 - PubMed
-
- Blundell JE, MacDiarmid JI. Fat as a risk factor for overconsumption: satiation, satiety, and patterns of eating. J Am Diet Assoc 97: S63–S69, 1997 - PubMed
-
- Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg 93: 210–215, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

