A randomized trial of 4-aminopyridine in EA2 and related familial episodic ataxias
- PMID: 21734179
- PMCID: PMC3136055
- DOI: 10.1212/WNL.0b013e318225ab07
A randomized trial of 4-aminopyridine in EA2 and related familial episodic ataxias
Abstract
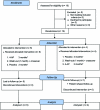
Objective: The therapeutic effects of 4-aminopyridine (4AP) were investigated in a randomized, double-blind, crossover trial in 10 subjects with familial episodic ataxia with nystagmus.
Methods: After randomization, placebo or 4AP (5 mg 3 times daily) was administered for 2 3-month-long treatment periods separated by a 1-month-long washout period. The primary outcome measure was the number of ataxia attacks per month; the secondary outcome measures were the attack duration and patient-reported quality of life (Vestibular Disorders Activities of Daily Living Scale [VDADL]). Nonparametric tests and a random-effects model were used for statistical analysis.
Results: The diagnosis of episodic ataxia type 2 (EA2) was genetically confirmed in 7 subjects. Patients receiving placebo had a median monthly attack frequency of 6.50, whereas patients taking 4AP had a frequency of 1.65 (p = 0.03). Median monthly attack duration decreased from 13.65 hours with placebo to 4.45 hours with 4AP (p = 0.08). The VDADL score decreased from 6.00 to 1.50 (p = 0.02). 4AP was well-tolerated.
Conclusions: This controlled trial on EA2 and familial episodic ataxia with nystagmus demonstrated that 4AP decreases attack frequency and improves quality of life.
Level of evidence: This crossover study provides Class II evidence that 4AP decreases attack frequency and improves the patient-reported quality of life in patients with episodic ataxia and related familial ataxias.
Figures


Comment in
-
News on ion channels: erythromelalgia, treatment of episodic ataxia and faciobrachial dystonic seizures.J Neurol. 2011 Sep;258(9):1734-6. doi: 10.1007/s00415-011-6215-6. J Neurol. 2011. PMID: 21847615 No abstract available.
-
A randomized trial of 4-aminopyridine in EA2 and related familial episodic ataxias.Neurology. 2011 Nov 29;77(22):1996-7; author reply 1997. doi: 10.1212/WNL.0b013e31823c162b. Neurology. 2011. PMID: 22123782 No abstract available.
References
-
- Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 1996;87:543–552 - PubMed
-
- Griggs RC, Moxley RT, 3rd, Lafrance RA, McQuillen J. Hereditary paroxysmal ataxia: response to acetazolamide. Neurology 1978;28:1259–1264 - PubMed
-
- Harno H, Hirvonen T, Kaunisto MA, et al. Acetazolamide improves neurotological abnormalities in a family with episodic ataxia type 2 (EA-2). J Neurol 2004;251:232–234 - PubMed
-
- Griggs RC, Nutt JG. Episodic ataxias as channelopathies. Ann Neurol 1995;37:285–287 - PubMed
-
- Jen JC, Graves TD, Hess EJ, Hanna MG, Griggs RC, Baloh RW. Primary episodic ataxias: diagnosis, pathogenesis and treatment. Brain 2007;130:2484–2493 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
