Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment
- PMID: 21734243
- PMCID: PMC3131055
- DOI: 10.1128/CMR.00057-10
Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment
Abstract
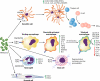
The discovery of Wolbachia intracellular bacteria within filarial nematodes, including Onchocerca volvulus, the causative agent of onchocerciasis or "river blindness," has delivered a paradigm shift in our understanding of the parasite's biology, to where we now know that the bacterial endosymbionts are essential for normal development of larvae and embryos and may support the long-term survival of adult worms. The apparent mutualistic dependency has also offered a novel approach to the treatment of onchocerciasis through the use of antibiotics to eliminate Wolbachia, delivering for the first time a treatment which has significant macrofilaricidal efficacy. Studies with other filarial nematode species have also highlighted a role for Wolbachia in transmission and infection of the mammalian host through a fascinating manipulation of mast cell-mediated vasodilation to enhance infectivity of vector-borne larvae. Wolbachia has also been identified as the principal driver of innate and adaptive Th1 inflammatory immunity, which can either contribute to disease pathogenesis or, with the Wolbachia-mediated recruitment of mast cells, enhance infectivity. The Wolbachia activation of innate inflammation also drives inflammatory adverse events in response to chemotherapy with either diethylcarbamazine (DEC) or ivermectin. In this review we summarize the experimental and field trial data which have uncovered the importance of Wolbachia symbiosis in onchocerciasis.
Figures


References
-
- Ali M. M., et al. 2003. Immune responses directed against microfilariae correlate with severity of clinical onchodermatitis and treatment history. J. Infect. Dis. 187:714–717 - PubMed
-
- Ali M. M., et al. 2007. Fc gamma RIIa (CD32) polymorphism and onchocercal skin disease: implications for the development of severe reactive onchodermatitis (ROD). Am. J. Trop. Med. Hyg. 77:1074–1078 - PubMed
-
- Baker R. H., Abdelnur O. M. 1986. Onchocerciasis in Sudan: the distribution of the disease and its vectors. Trop. Med. Parasitol. 37:341–355 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

