Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum
- PMID: 21734244
- PMCID: PMC3131063
- DOI: 10.1128/CMR.00064-10
Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum
Abstract
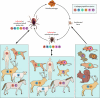
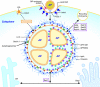
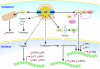
Anaplasma phagocytophilum persists in nature by cycling between mammals and ticks. Human infection by the bite of an infected tick leads to a potentially fatal emerging disease called human granulocytic anaplasmosis. A. phagocytophilum is an obligatory intracellular bacterium that replicates inside mammalian granulocytes and the salivary gland and midgut cells of ticks. A. phagocytophilum evolved the remarkable ability to hijack the regulatory system of host cells. A. phagocytophilum alters vesicular traffic to create an intracellular membrane-bound compartment that allows replication in seclusion from lysosomes. The bacterium downregulates or actively inhibits a number of innate immune responses of mammalian host cells, and it upregulates cellular cholesterol uptake to acquire cholesterol for survival. It also upregulates several genes critical for the infection of ticks, and it prolongs tick survival at freezing temperatures. Several host factors that exacerbate infection have been identified, including interleukin-8 (IL-8) and cholesterol. Host factors that overcome infection include IL-12 and gamma interferon (IFN-γ). Two bacterial type IV secretion effectors and several bacterial proteins that associate with inclusion membranes have been identified. An understanding of the molecular mechanisms underlying A. phagocytophilum infection will foster the development of creative ideas to prevent or treat this emerging tick-borne disease.
Figures

References
-
- Reference deleted.
-
- Aguero-Rosenfeld M. E., et al. 1996. Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann. Intern. Med. 125:904–908 - PubMed
-
- Ahkee S., Ramirez J. 1996. A case of concurrent Lyme meningitis with ehrlichiosis. Scand. J. Infect. Dis. 28:527–528 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

