Pathogenesis of chagas' disease: parasite persistence and autoimmunity
- PMID: 21734249
- PMCID: PMC3131057
- DOI: 10.1128/CMR.00063-10
Pathogenesis of chagas' disease: parasite persistence and autoimmunity
Abstract
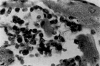
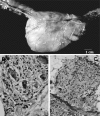
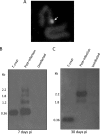
Acute Trypanosoma cruzi infections can be asymptomatic, but chronically infected individuals can die of Chagas' disease. The transfer of the parasite mitochondrial kinetoplast DNA (kDNA) minicircle to the genome of chagasic patients can explain the pathogenesis of the disease; in cases of Chagas' disease with evident cardiomyopathy, the kDNA minicircles integrate mainly into retrotransposons at several chromosomes, but the minicircles are also detected in coding regions of genes that regulate cell growth, differentiation, and immune responses. An accurate evaluation of the role played by the genotype alterations in the autoimmune rejection of self-tissues in Chagas' disease is achieved with the cross-kingdom chicken model system, which is refractory to T. cruzi infections. The inoculation of T. cruzi into embryonated eggs prior to incubation generates parasite-free chicks, which retain the kDNA minicircle sequence mainly in the macrochromosome coding genes. Crossbreeding transfers the kDNA mutations to the chicken progeny. The kDNA-mutated chickens develop severe cardiomyopathy in adult life and die of heart failure. The phenotyping of the lesions revealed that cytotoxic CD45, CD8(+) γδ, and CD8α(+) T lymphocytes carry out the rejection of the chicken heart. These results suggest that the inflammatory cardiomyopathy of Chagas' disease is a genetically driven autoimmune disease.
Figures
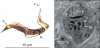








Similar articles
-
Parasite induced genetically driven autoimmune Chagas heart disease in the chicken model.J Vis Exp. 2012 Jul 29;(65):3716. doi: 10.3791/3716. J Vis Exp. 2012. PMID: 22951533 Free PMC article.
-
Trypanosoma cruzi in the chicken model: Chagas-like heart disease in the absence of parasitism.PLoS Negl Trop Dis. 2011 Mar 29;5(3):e1000. doi: 10.1371/journal.pntd.0001000. PLoS Negl Trop Dis. 2011. PMID: 21468314 Free PMC article.
-
Inhibition of autoimmune Chagas-like heart disease by bone marrow transplantation.PLoS Negl Trop Dis. 2014 Dec 18;8(12):e3384. doi: 10.1371/journal.pntd.0003384. eCollection 2014 Dec. PLoS Negl Trop Dis. 2014. PMID: 25521296 Free PMC article.
-
Trypanosoma cruzi-induced molecular mimicry and Chagas' disease.Curr Top Microbiol Immunol. 2005;296:89-123. doi: 10.1007/3-540-30791-5_6. Curr Top Microbiol Immunol. 2005. PMID: 16323421 Review.
-
Evolution and pathology in chagas disease--a review.Mem Inst Oswaldo Cruz. 2006 Aug;101(5):463-91. doi: 10.1590/s0074-02762006000500001. Mem Inst Oswaldo Cruz. 2006. PMID: 17072450 Review.
Cited by
-
Parasite induced genetically driven autoimmune Chagas heart disease in the chicken model.J Vis Exp. 2012 Jul 29;(65):3716. doi: 10.3791/3716. J Vis Exp. 2012. PMID: 22951533 Free PMC article.
-
Pathology and Pathogenesis of Chagas Heart Disease.Annu Rev Pathol. 2019 Jan 24;14:421-447. doi: 10.1146/annurev-pathol-020117-043711. Epub 2018 Oct 24. Annu Rev Pathol. 2019. PMID: 30355152 Free PMC article. Review.
-
Potential Application of Exosomes in Vaccine Development and Delivery.Pharm Res. 2022 Nov;39(11):2635-2671. doi: 10.1007/s11095-021-03143-4. Epub 2022 Jan 13. Pharm Res. 2022. PMID: 35028802 Free PMC article. Review.
-
Differential Regulation of L-Arginine Metabolism through NOS2 and Arginases during Infection with Trypanosoma cruzi.Pathogens. 2024 Oct 8;13(10):878. doi: 10.3390/pathogens13100878. Pathogens. 2024. PMID: 39452749 Free PMC article.
-
Mast cells in the colon of Trypanosoma cruzi-infected patients: are they involved in the recruitment, survival and/or activation of eosinophils?Parasitol Res. 2015 May;114(5):1847-56. doi: 10.1007/s00436-015-4371-9. Epub 2015 Feb 25. Parasitol Res. 2015. PMID: 25711147
References
-
- Abel L. C., et al. 2005. T cell epitope characterization in tandemly repetitive Trypanosoma cruzi B13 protein. Microbes Infect. 7:1184–1195 - PubMed
-
- Abrahamsohn I. A., Silva W. D. 1977. Antibody dependent cell-mediated cytotoxicity against Trypanosoma cruzi. Parasitology 75:317–323 - PubMed
-
- Acosta A. M., Santos-Buch C. A. 1985. Autoimmune myocarditis induced by Trypanosoma cruzi. Circulation 71:1255–1261 - PubMed
-
- Acosta-Serrano A., Almeida I. C., Freitas L. H., Jr., Yoshida N., Schenkman S. 2001. The mucin-like glycoprotein super-family of Trypanosoma cruzi: structure and biological roles. Mol. Biochem. Parasitol. 114:143–150 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

