Generation of colonies of induced trophoblast cells during standard reprogramming of porcine fibroblasts to induced pluripotent stem cells
- PMID: 21734265
- PMCID: PMC3184293
- DOI: 10.1095/biolreprod.111.092809
Generation of colonies of induced trophoblast cells during standard reprogramming of porcine fibroblasts to induced pluripotent stem cells
Abstract
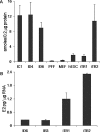
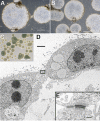
During reprogramming of porcine mesenchymal cells with a four-factor (POU5F1/SOX2/KLF4/MYC) mixture of vectors, a fraction of the colonies had an atypical phenotype and arose earlier than the recognizable porcine induced pluripotent stem (iPS) cell colonies. Within days after each passage, patches of cells with an epithelial phenotype formed raised domes, particularly under 20% O(2) conditions. Relative to gene expression of the iPS cells, there was up-regulation of genes for transcription factors associated with trophoblast (TR) lineage emergence, e.g., GATA2, PPARG, MSX2, DLX3, HAND1, GCM1, CDX2, ID2, ELF5, TCFAP2C, and TEAD4 and for genes required for synthesis of products more typical of differentiated TR, such as steroids (HSD17B1, CYP11A1, and STAR), pregnancy-associated glycoproteins (PAG6), and select cytokines (IFND, IFNG, and IL1B). Although POU5F1 was down-regulated relative to that in iPS cells, it was not silenced in the induced TR (iTR) cells over continued passage. Like iPS cells, iTR cells did not senesce on extended passage and displayed high telomerase activity. Upon xenografting into immunodeficient mice, iTR cells formed nonhemorrhagic teratomas composed largely of layers of epithelium expressing TR markers. When cultured under conditions that promoted embryoid body formation, iTR cells formed floating spheres consisting of a single epithelial sheet whose cells were tethered laterally by desmosome-like structures. In conclusion, reprogramming of porcine fibroblasts to iPS cells generates, as a by-product, colonies composed of self-renewing populations of TR cells, possibly containing TR stem cells.
Figures



Similar articles
-
Reprogramming in vivo produces teratomas and iPS cells with totipotency features.Nature. 2013 Oct 17;502(7471):340-5. doi: 10.1038/nature12586. Epub 2013 Sep 11. Nature. 2013. PMID: 24025773
-
Generation of induced pluripotent stem cells from buffalo (Bubalus bubalis) fetal fibroblasts with buffalo defined factors.Stem Cells Dev. 2012 Sep 1;21(13):2485-94. doi: 10.1089/scd.2012.0018. Epub 2012 May 14. Stem Cells Dev. 2012. PMID: 22420535
-
Derivation of induced pluripotent stem cells from pig somatic cells.Proc Natl Acad Sci U S A. 2009 Jul 7;106(27):10993-8. doi: 10.1073/pnas.0905284106. Epub 2009 Jun 18. Proc Natl Acad Sci U S A. 2009. PMID: 19541600 Free PMC article.
-
Generation of induced pluripotent stem cells by efficient reprogramming of adult bone marrow cells.Stem Cells Dev. 2010 Feb;19(2):229-38. doi: 10.1089/scd.2009.0149. Stem Cells Dev. 2010. PMID: 19558219
-
Induced pluripotent stem cells from swine (Sus scrofa): why they may prove to be important.Cell Cycle. 2009 Oct 1;8(19):3078-81. doi: 10.4161/cc.8.19.9589. Epub 2009 Oct 21. Cell Cycle. 2009. PMID: 19738434 Review.
Cited by
-
Cell-cell communication-mediated cell-type-specific parent-of-origin effects in mammals.Nat Commun. 2025 Jun 2;16(1):5106. doi: 10.1038/s41467-025-60469-y. Nat Commun. 2025. PMID: 40456771 Free PMC article.
-
GATA Transcription Factors in Pregnancy.Med J Obstet Gynecol. 2013 Oct 18;1(2):1013. Med J Obstet Gynecol. 2013. PMID: 25664333 Free PMC article.
-
Comparison of Placental HSD17B1 Expression and Its Regulation in Various Mammalian Species.Animals (Basel). 2023 Feb 10;13(4):622. doi: 10.3390/ani13040622. Animals (Basel). 2023. PMID: 36830409 Free PMC article.
-
Cellular reprogramming in farm animals: an overview of iPSC generation in the mammalian farm animal species.J Anim Sci Biotechnol. 2016 Feb 19;7:10. doi: 10.1186/s40104-016-0070-3. eCollection 2016. J Anim Sci Biotechnol. 2016. PMID: 26900466 Free PMC article. Review.
-
The Efficient Derivation of Trophoblast Cells from Porcine In Vitro Fertilized and Parthenogenetic Blastocysts and Culture with ROCK Inhibitor Y-27632.PLoS One. 2015 Nov 10;10(11):e0142442. doi: 10.1371/journal.pone.0142442. eCollection 2015. PLoS One. 2015. PMID: 26555939 Free PMC article.
References
-
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science 1998; 282: 2072 2075 - PubMed
-
- Vandevoort CA, Thirkill TL, Douglas GC. Blastocyst-derived trophoblast stem cells from the rhesus monkey. Stem Cells Dev 2007; 16: 779 788 - PubMed
-
- Tanaka S. Derivation and culture of mouse trophoblast stem cells in vitro. Methods Mol Biol 2006; 329: 35 44 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

